Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) \(n_{Zn}=\dfrac{16,25}{65}=0,25\left(mol\right)\)
PTHH: Zn + 2HCl --> ZnCl2 + H2
0,25-------------------->0,25
=> VH2 = 0,25.22,4 = 5,6 (l)
b)
PTHH: CuO + H2 --to--> Cu + H2O
0,25--->0,25
=> mCu = 0,25.64 = 16 (g)

Fe + 2HCl -> FeCl2 + H2
nFe = 5,6/56 = 0,1 mol
=>nH2 = 0,1 mol
=> VH2= 0,1*22,4= 2,24 lít
\(n_{Fe}=\dfrac{5,6}{56}=0,1\left(mol\right)\)
PTHH: Fe + 2HCl ---> FeCl2 + H2
0,1-->0,2------------------>0,1
=> \(\left\{{}\begin{matrix}m_{ddHCl}=\dfrac{0,2.36,5}{15\%}=\dfrac{146}{3}\left(g\right)\\V_{H_2}=0,1.22,4=4,48\left(l\right)\end{matrix}\right.\)

\(n_{Zn}=\dfrac{m}{M}=\dfrac{16,25}{65}=0,25\left(mol\right)\\ PTHH:Zn+H_2SO_4->ZnSO_4+H_2\)
tỉ lệ 1 : 1 : 1 : 1
n(mol) 0,25-->0,25------->0,25------>0,25
\(V_{H_2\left(dktc\right)}=n\cdot22,4=0,25\cdot22,4=5,6\left(l\right)\\ m_{ZnSO_4}=n\cdot M=0,25\cdot\left(65+32+16\cdot4\right)=40,25\left(g\right)\)

a. \(PTHH:Mg+2HCl\rightarrow MgCl_2+H_2\uparrow\)
\(n_{Mg}=\dfrac{m_{Mg}}{M_{Mg}}=\dfrac{12}{24}=0,5\left(mol\right)\)
- Mol theo PTHH : \(1:2:1:1\)
- Mol theo phản ứng : \(0,5\rightarrow1\rightarrow0,5\rightarrow0,5\)
\(\Rightarrow n_{H_2}=0,5\left(mol\right)\)
\(\Rightarrow V_{H_2}=n_{H_2}.22,4=0,5.22,4=11,2\left(l\right)\)
b. Từ a. \(\Rightarrow n_{HCl}=1\left(mol\right)\)
\(\Rightarrow m_{HCl}=n_{HCl}.M_{HCl}=1.\left(1+35,5\right)=36,5\left(g\right)\)
c. \(n_{O_2}=\dfrac{V_{O_2}}{22,4}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
\(PTHH:2H_2+O_2\underrightarrow{t^o}2H_2O\)
- Mol theo PTHH : \(2:1:2\)
- Mol theo phản ứng : \(0,6\leftarrow0,3\rightarrow0,6\)
\(\Rightarrow n_{H_2O}=0,6\left(mol\right)\)
\(\Rightarrow m_{H_2O}=n_{H_2O}.M_{H_2O}=0,6.\left(2+16\right)=10,8\left(g\right)\)

Zn+2HCl->Zncl2+H2
0,4----0,8----0,4----0,4
n Zn=0,4 mol
VH2=0,4.22,4=8,96l
m ZnCl2=0,4.136=54,4g
2H2+O2-to>2H2O
0,4------0,2----0,4
n O2=0,2 mol
=>pứ hết
=>m H2O=0,4.18=7,2g
a.b.\(n_{Zn}=\dfrac{26}{65}=0,4mol\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
0,4 0,4 0,4 ( mol )
\(m_{ZnCl_2}=0,4.136=54,4g\)
\(V_{H_2}=0,4.22,4=8,96l\)
c.\(n_{O_2}=\dfrac{4,48}{22,4}=0,2mol\)
\(2H_2+O_2\rightarrow\left(t^o\right)2H_2O\)
0,4 = 0,2 ( mol )
0,4 0,2 0,4 ( mol )
\(m_{H_2O}=0,4.18=7,2g\)

a.b.\(n_{Mg}=\dfrac{3,6}{24}=0,15mol\)
\(Mg+2HCl\rightarrow MgCl_2+H_2\)
0,15 0,3 0,15 ( mol )
\(V_{H_2}=0,15.22,4=3,36l\)
\(m_{HCl}=0,3.36,5=10,95g\)
c.\(n_{H_2}=0,15.60\%=0,09mol\)
\(Ag_2O+H_2\rightarrow\left(t^o\right)2Ag+H_2O\)
0,09 0,18 ( mol )
\(m_{Ag}=0,18.108=19,44g\)
\(a.n_{Mg}=\dfrac{3,6}{24}=0,15\left(mol\right)\\ Mg+2HCl\rightarrow MgCl_2+H_2\\ TheoPT:n_{H_2}=n_{Mg}=0,15\left(mol\right)\\ \Rightarrow V_{H_2}=0,15.22,4=3,36\left(l\right)\\ b.TheoPT:n_{HCl}=2n_{Mg}=0,3\left(mol\right)\\ \Rightarrow m_{Mg}=0,3.36,5=10,95\left(g\right)\\ c.n_{H_2\left(pứ\right)}=0,15.60\%=0,054\left(g\right)\\ H_2+Ag_2O-^{t^o}\rightarrow2Ag+H_2O\\ n_{Ag}=2n_{H_2}=0,108\left(mol\right)\\ \Rightarrow m_{Ag}=0,108.108=11,664\left(g\right)\)

\(n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\)
PTHH: Zn + 2HCl --> ZnCl2 + H2
0,2------------------>0,2
=> nH2(dùng để khử) = 0,4 (mol)
PTHH: PbO + H2 --to--> Pb + H2O
0,4------->0,4
=> mPb = 0,4.207 = 82,8 (g)
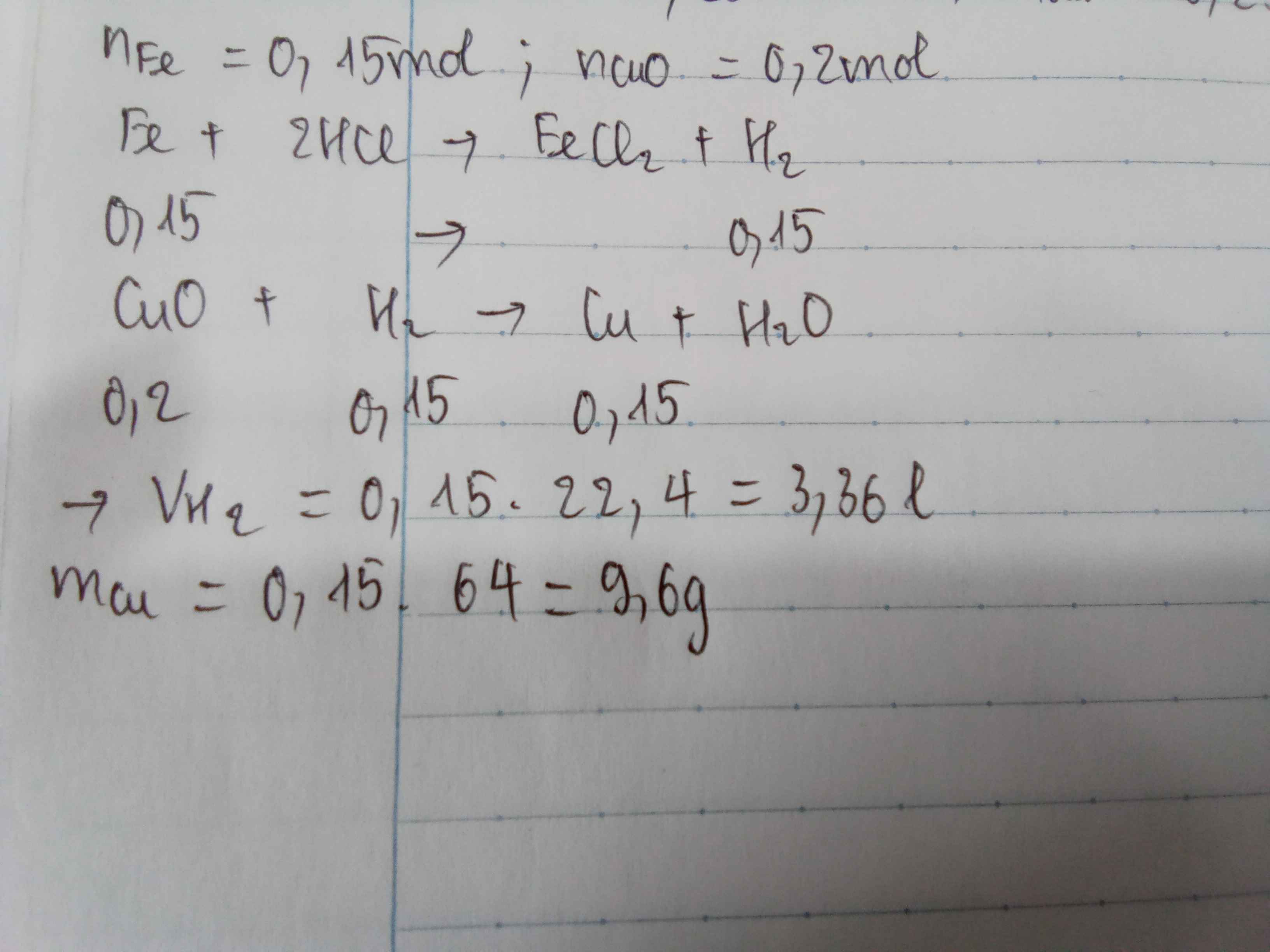


a) \(n_{Zn}=\dfrac{16,25}{65}=0,25\left(mol\right)\)
PTHH: Zn + 2HCl --> ZnCl2 + H2
0,25----------------->0,25
=> VH2 = 0,25.22,4 = 5,6 (l)
b)
\(n_{CuO}=\dfrac{16}{80}=0,2\left(mol\right)\)
PTHH: CuO + H2 --to--> Cu + H2O
Xét tỉ lệ: \(\dfrac{0,2}{1}< \dfrac{0,25}{1}\) => CuO hết, H2 dư
PTHH: CuO + H2 --to--> Cu + H2O
0,2-------------->0,2
=> mCu = 0,2.64 = 12,8 (g)
em cảm ơn