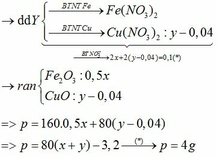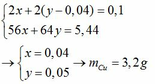Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) mNaOH= 200.20%= 40(g)
=>nNaOH=1(mol)
PTHH: 2 NaOH + CuCl2 -> 2 NaCl + Cu(OH)2
Dung dịch sau khi lọc kết tủa có NaCl.
nNaCl=nNaOH= 1(mol)
nCuCl2=nCu(OH)2=nNaOH/2=1/2=0,5(mol)
mNaCl=1.58,5=58,5(g)
mCuCl2=0,5.135=67,5(g)
=> mddCuCl2=(67,5.100)/10=675(g)
mCu(OH)2=0,5.98=49(g)
=>mddNaCl=mddNaOH+ mddCuCl2 - mCu(OH)2= 200+675 - 98=777(g)
=> \(C\%ddNaCl=\dfrac{58,5}{777}.100\approx7,529\%\)
b) PTHH: Cu(OH)2 -to-> CuO + H2O
0,5__________________0,5(mol)
m(rắn)=mCuO=0,5.80=4(g)

\(n_{AlCl_3}=0.2\cdot1=0.2\left(mol\right)\)
\(n_{NaOH}=0.5V\left(mol\right)\)
\(n_{Al_2O_3}=\dfrac{5.1}{102}=0.05\left(mol\right)\)
\(2Al\left(OH\right)_3\underrightarrow{^{^{t^0}}}Al_2O_3+3H_2O\)
\(0.1...............0.05\)
TH1 : Al(OH)3 không bị hòa tan.
\(AlCl_3+3NaOH\rightarrow Al\left(OH\right)_3+3NaCl\)
\(0.1...........0.3................0.1\)
\(\Leftrightarrow V=\dfrac{0.3}{0.5}=0.6\left(l\right)\)
TH2 : Al(OH)3 bị hòa tan một phần
\(AlCl_3+3NaOH\rightarrow Al\left(OH\right)_3+3NaCl\)
\(0.2...........0.6................0.2\)
\(NaOH+Al\left(OH\right)_3\rightarrow NaAlO_2+2H_2O\)
\(0.5V-0.6...0.5V-0.6\)
\(n_{Al\left(OH\right)_3}=0.2+0.5V-0.6=0.1\left(mol\right)\)
\(\Rightarrow V=1\left(l\right)\)

\(CuCl_2+2NaOH-->Cu\left(OH\right)_2\downarrow+2NaCl\left(1\right)\)
0,3_________0,6__________0,3
\(Cu\left(OH\right)_2--to->CuO+H_2O\left(2\right)\)
0,3_________________0,3
\(n_{NaOH}=\frac{32}{40}=0,8\left(mol\right)\)
=> NaOH dư
a) \(n_{CuO}=0,3\left(mol\right)=>m_{CuO}=0,3.80=24\left(g\right)\)
b) \(n_{NaOH}\) dư =0,8-0,6=0,2(mol)
=> \(m_{NaOH}\)dư=0,2.40=20(g)

\(n_{CuSO_4}=\dfrac{200.16\%}{160}=0,2\left(mol\right)\)
PTHH :
\(CuSO_4+2NaOH\rightarrow Cu\left(OH\right)_2\downarrow+Na_2SO_4\)
0,2 0,4 0,2 0,2
\(m_{NaOH}=0,4.40=16\left(g\right)\)
\(m_{ddNaOH}=\dfrac{16.100}{10}=160\left(g\right)\)
\(c,m_{Na_2SO_4}=0,2.142=28,4\left(g\right)\)
\(m_{ddNa_2SO_4}=200+160-\left(0,2.98\right)=340,4\left(g\right)\)
\(C\%_{Na_2SO_4}=\dfrac{28,4}{240,4}.100\%\approx8,34\%\)
\(d,PTHH:\)
\(Cu\left(OH\right)_2\underrightarrow{t^o}CuO+H_2O\)
0,2 0,2
\(m_{CuO}=0,2.80=16\left(g\right)\)
a, \(CuSO_4+2NaOH\rightarrow Cu\left(OH\right)_2+Na_2SO_4\)
b, \(m_{CuSO_4}=200.16\%=32\left(g\right)\Rightarrow n_{CuSO_4}=\dfrac{32}{160}=0,2\left(mol\right)\)
\(n_{NaOH}=2n_{CuSO_4}=0,4\left(mol\right)\Rightarrow m_{ddNaOH}=\dfrac{0,4.40}{10\%}=160\left(g\right)\)
c, \(n_{Cu\left(OH\right)_2}=n_{Na_2SO_4}=n_{CuSO_4}=0,2\left(mol\right)\)
\(\Rightarrow C\%_{Na_2SO_4}=\dfrac{0,2.142}{200+160-0,2.98}.100\%\approx8,34\%\)
d, \(Cu\left(OH\right)_2\underrightarrow{t^o}CuO+H_2O\)
\(n_{CuO}=n_{Cu\left(OH\right)_2}=0,2\left(mol\right)\Rightarrow m_{CuO}=0,2.80=16\left(g\right)\)

a) $n_{Fe} = \dfrac{11,2}{56} = 0,2(mol) ; n_{HCl} = \dfrac{300.3,65\%}{36,5} = 0,3(mol)$
$Fe + 2HCl \to FeCl_2 + H_2$
Ta thấy :
$n_{Fe} :1 > n_{HCl} : 2$ nên Fe dư
$n_{H_2} = \dfrac{1}{2}n_{HCl} = 0,15(mol)$
$V_{H_2} = 0,15.22,4 = 3,36(lít)$
b) $n_{FeCl_2} = \dfrac{1}{2}n_{HCl} = 0,15(mol)$
$FeCl_2 + 2NaOH \to Fe(OH)_2 + 2NaCl$
$4Fe(OH)_2 +O_2 \xrightarrow{t^o} 2Fe_2O_3 + 4H_2O$
$n_{Fe_2O_3} = \dfrac{1}{2}n_{FeCl_2} = 0,075(mol)$
$m = 0,075.160 = 12(gam)$