Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(1,2H_2+O_2\underrightarrow{t}2H_2O\)
\(2Mg+O_2\underrightarrow{t}2MgO\)
\(2Cu+O_2\underrightarrow{t}2CuO\)
\(S+O_2\underrightarrow{t}SO_2\)
\(4Al+3O_2\underrightarrow{t}2Al_2O_3\)
\(C+O_2\underrightarrow{t}CO_2\)
\(4P+5O_2\underrightarrow{t}2P_2O_5\)
\(2,PTHH:C+O_2\underrightarrow{t}CO_2\)
\(a,n_{O_2}=0,2\left(mol\right)\Rightarrow n_{CO_2}=0,2\left(mol\right)\Rightarrow m_{CO_2}=8,8\left(g\right)\)
\(b,n_C=0,3\left(mol\right)\Rightarrow n_{CO_2}=0,3\left(mol\right)\Rightarrow m_{CO_2}=13,2\left(g\right)\)
c, Vì\(\frac{0,3}{1}>\frac{0,2}{1}\)nên C phản ửng dư, O2 phản ứng hết, Bài toán tính theo O2
\(n_{O_2}=0,2\left(mol\right)\Rightarrow n_{CO_2}=0,2\left(mol\right)\Rightarrow m_{CO_2}=8,8\left(g\right)\)
\(3,PTHH:CH_4+2O_2\underrightarrow{t}CO_2+2H_2O\)
\(C_2H_2+\frac{5}{2}O_2\underrightarrow{t}2CO_2+H_2O\)
\(C_2H_6O+3O_2\underrightarrow{t}2CO_2+3H_2O\)
\(4,a,PTHH:4P+5O_2\underrightarrow{t}2P_2O_5\)
\(n_P=1,5\left(mol\right)\Rightarrow n_{O_2}=1,2\left(mol\right)\Rightarrow m_{O_2}=38,4\left(g\right)\)
\(b,PTHH:C+O_2\underrightarrow{t}CO_2\)
\(n_C=2,5\left(mol\right)\Rightarrow n_{O_2}=2,5\left(mol\right)\Rightarrow m_{O_2}=80\left(g\right)\)
\(c,PTHH:4Al+3O_2\underrightarrow{t}2Al_2O_3\)
\(n_{Al}=2,5\left(mol\right)\Rightarrow n_{O_2}=1,875\left(mol\right)\Rightarrow m_{O_2}=60\left(g\right)\)
\(d,PTHH:2H_2+O_2\underrightarrow{t}2H_2O\)
\(TH_1:\left(đktc\right)n_{H_2}=1,5\left(mol\right)\Rightarrow n_{O_2}=0,75\left(mol\right)\Rightarrow m_{O_2}=24\left(g\right)\)
\(TH_2:\left(đkt\right)n_{H_2}=1,4\left(mol\right)\Rightarrow n_{O_2}=0,7\left(mol\right)\Rightarrow m_{O_2}=22,4\left(g\right)\)
\(5,PTHH:S+O_2\underrightarrow{t}SO_2\)
\(n_{O_2}=0,46875\left(mol\right)\)
\(n_{SO_2}=0,3\left(mol\right)\)
Vì\(0,46875>0,3\left(n_{O_2}>n_{SO_2}\right)\)nên S phản ứng hết, bài toán tính theo S.
\(a,\Rightarrow n_S=n_{SO_2}=0,3\left(mol\right)\Rightarrow m_S=9,6\left(g\right)\)
\(n_{O_2}\left(dư\right)=0,16875\left(mol\right)\Rightarrow m_{O_2}\left(dư\right)=5,4\left(g\right)\)
\(6,a,PTHH:C+O_2\underrightarrow{t}CO_2\)
\(n_{O_2}=1,5\left(mol\right)\Rightarrow n_C=1,5\left(mol\right)\Rightarrow m_C=18\left(g\right)\)
\(b,PTHH:2H_2+O_2\underrightarrow{t}2H_2O\)
\(n_{O_2}=1,5\left(mol\right)\Rightarrow n_{H_2}=0,75\left(mol\right)\Rightarrow m_{H_2}=1,5\left(g\right)\)
\(c,PTHH:S+O_2\underrightarrow{t}SO_2\)
\(n_{O_2}=1,5\left(mol\right)\Rightarrow n_S=1,5\left(mol\right)\Rightarrow m_S=48\left(g\right)\)
\(d,PTHH:4P+5O_2\underrightarrow{t}2P_2O_5\)
\(n_{O_2}=1,5\left(mol\right)\Rightarrow n_P=1,2\left(mol\right)\Rightarrow m_P=37,2\left(g\right)\)
\(7,n_{O_2}=5\left(mol\right)\Rightarrow V_{O_2}=112\left(l\right)\left(đktc\right)\);\(V_{O_2}=120\left(l\right)\left(đkt\right)\)
\(8,PTHH:C+O_2\underrightarrow{t}CO_2\)
\(m_C=0,96\left(kg\right)\Rightarrow n_C=0,08\left(kmol\right)=80\left(mol\right)\Rightarrow n_{O_2}=80\left(mol\right)\Rightarrow V_{O_2}=1792\left(l\right)\)
\(9,n_p=0,2\left(mol\right);n_{O_2}=0,3\left(mol\right)\)
\(PTHH:4P+5O_2\underrightarrow{t}2P_2O_5\)
Vì\(\frac{0,2}{4}< \frac{0,3}{5}\)nên P hết O2 dư, bài toán tính theo P.
\(a,n_{O_2}\left(dư\right)=0,05\left(mol\right)\Rightarrow m_{O_2}\left(dư\right)=1,6\left(g\right)\)
\(b,n_{P_2O_5}=0,1\left(mol\right)\Rightarrow m_{P_2O_5}=14,2\left(g\right)\)

nP = 6.2/31 = 0.2 (mol)
nO2 = 6.72/22.4 = 0.3 (mol)
4P + 5O2 -to-> 2P2O5
0.2___0.25_____0.1
mO2 dư = ( 0.3 - 0.25) * 32 = 1.6(g)
mP2O5 = 0.1*142 = 14.2 (g)
Ta có: \(n_P=\dfrac{6.2}{31}=0.29mol\)
\(n_{O_2}=\dfrac{6.72}{22.4}=0.3mol\)
PTHH:
\(4P+5O_2\underrightarrow{t^o}2P_2O_5\)
ta có:
\(\left\{{}\begin{matrix}\dfrac{n_{P\left(bra\right)}}{nP_{\left(pthh\right)}}=\dfrac{0.2}{4}=0.05\\\dfrac{n_{O_2\left(bra\right)}}{n_{O_2}\left(pthh\right)}=\dfrac{0.3}{5}=0.06\end{matrix}\right.\)
=> \(O_2\) dư
\(4P+5O_2\underrightarrow{t^o}2P_2O_5\)
4 ----------->2
0.2---------->0.1=nP2O5
=>\(m_{P_2O_5}=142.0.1=14.2\left(g\right)\)

\(n_{hhkhí}=\dfrac{16,8}{22,4}=0,75\left(mol\right)\)
Gọi \(n_{SO_2}=a\left(mol\right)\left(0< a< 0,75\right)\)
\(\rightarrow n_{O_2\left(dư\right)}=0,75-b\left(mol\right)\)
Ta có: \(\dfrac{64a+32\left(0,75-a\right)}{0,75}=\dfrac{33,6}{1}=33,6\left(\dfrac{g}{mol}\right)\)
\(\rightarrow a=0,0375\left(mol\right)\)
\(\rightarrow\left\{{}\begin{matrix}\%V_{SO_2}=\dfrac{0,0375}{0,75}=5\%\\\%V_{O_2\left(dư\right)}=100\%-5\%=95\%\end{matrix}\right.\)

\(n_P=\dfrac{6,2}{31}=0,2\left(mol\right)\\ n_{O_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\\ PTHH:4P+5O_2\underrightarrow{t^o}2P_2O_5\\ LTL:\dfrac{0,2}{4}< \dfrac{0,4}{5}\Rightarrow O_2dư\)
\(n_{O_2\left(pư\right)}=\dfrac{5}{4}n_P=\dfrac{5}{4}.0,2=0,25\left(mol\right)\\ n_{O_2\left(dư\right)}=0,4-0,25=0,15\left(mol\right)\)
\(n_{P_2O_5\left(lt\right)}=\dfrac{1}{2}n_P=\dfrac{1}{2}.0,2=0,1\left(mol\right)\\ m_{P_2O_5\left(lt\right)}=0,1.142=14,2\left(g\right)\\ m_{P_2O_5\left(tt\right)}=0,1.142.80\%=11,36\left(g\right)\)

\(n_P=\dfrac{12,4}{31}=0,4\left(mol\right)\\
pthh:4P+5O_2\underrightarrow{t^o}2P_2O_5\)
0,4 0,2
\(m_{P_2O_5}=142.0,2=28,4g\)
\(n_{O_2}=\dfrac{17}{32}=0,53\left(mol\right)\)
\(pthh:4P+5O_2\underrightarrow{t^o}2P_2O_5\\
LTL:\dfrac{0,4}{4}< \dfrac{0,53}{5}\)
=> O2 dư
\(n_{O_2\left(p\text{ư}\right)}=\dfrac{5}{4}n_P=0,5\left(mol\right)\\
m_{O_2\left(d\right)}=\left(0,53-0,5\right).32=0,96g\)
`4P + 5O_2` $\xrightarrow[]{t^o}$ `2P_2 O_5`
`0,4` `0,5` `0,2` `(mol)`
`n_P = [ 12,4 ] / 31 = 0,4 (mol)`
`a) m_[P_2 O_5] = 0,2 . 142 = 28,4 (g)`
`b) n_[O_2] = 17 / 32 = 0,53125 (mol)`
Ta có: `[ 0,4 ] / 4 < [ 0,53125 ] / 5`
`->O_2` dư
`=> m_[O_2 (dư)] = ( 0,53125 - 0,5 ) . 32 = 1(g)`

nP = 2,48/31 = 0,08 (mol)
PTHH: 4P + 5O2 -> (t°) 2P2O5
Mol: 0,08 ---> 0,1 ---> 0,04
mP2O5 = 0,04 . 142 = 5,68 (g)
b) nO2 = 4/32 = 0,125 (mol)
So sánh: 0,125 > 0,1 => O2 dư
nO2 (dư) = 0,125 - 0,1 = 0,025 (mol)
mO2 (dư) = 0,025 . 32 = 0,8 (g)

nP=\(\dfrac{62}{31}\)=0,2(mol)
nO2=\(\dfrac{7,84}{22,4}\)=0,35(mol)
PTHH:4P+5O2to→2P2O5
tpứ: 0,2 0,35
pứ: 0,2 0,25 0,1
spứ: 0 0,1 0,1
a)chất còn dư là oxi
mO2dư=0,1.32=3,2(g)
b)mP2O5=n.M=0,1.142=14,2(g)
\(a.n_P=0,2\left(mol\right);n_{O_2}=0,35\left(mol\right)\\ 4P+5O_2-^{t^o}\rightarrow2P_2O_5\\ LTL:\dfrac{0,2}{4}< \dfrac{0,35}{5}\\ \Rightarrow SauphảnứngO_2dư\\ n_{O_2\left(pứ\right)}=\dfrac{5}{4}n_P=0,25\left(mol\right)\\ \Rightarrow m_{P\left(dư\right)}=\left(0,35-0,25\right).32=3,2\left(g\right)\\ b.n_{P_2O_5}=\dfrac{1}{2}n_P=0,1\left(mol\right)\\ \Rightarrow m_{P_2O_5}=0,1.142=14,2\left(g\right)\)

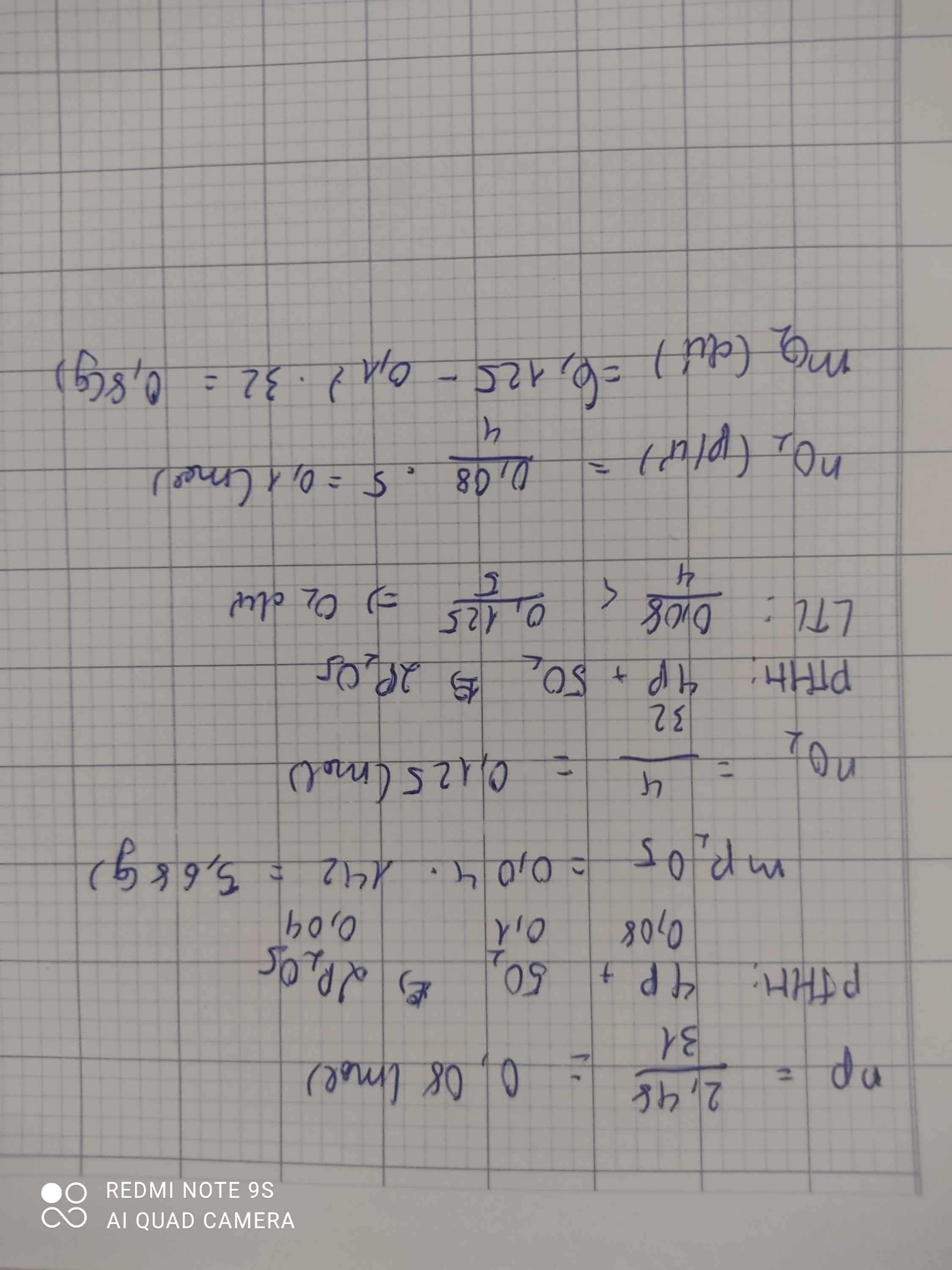
Câu 1:
\(\left\{{}\begin{matrix}n_P=\frac{6,2}{32}=0,2\left(mol\right)\\n_{O2}=\frac{6,72}{22,4}=0,3\left(mol\right)\end{matrix}\right.\)
\(4P+5O_2\rightarrow2P_2O_5\)
Vì 0,2 < 0,3
Nên O2 dư
\(\Rightarrow m_{O2\left(dư\right)}=0,2.\left(2.16\right)=9,6\left(g\right)\)
The PTHH:
\(n_{P2O5}=\frac{1}{2}n_P=0,1\left(mol\right)\)
\(\Rightarrow m_{P2O5}=0,1.144=14,4\left(g\right)\)
Câu 2:
Ta có:
\(n_{SO2}=\frac{19,2}{32+16.2}=0,3\left(mol\right);n_{O2}=0,46875\left(mol\right)\)
\(S+O_2\rightarrow SO_2\)
Theo PTHH:
\(n_{O2\left(pư\right)}=n_{S\left(pư\right)}=n_{SO2}\)
Nên O2 dư, S hết.
\(\Rightarrow n_S=n_{SO2}=0,3\left(mol\right)\Rightarrow m_S=0,3.32=9,6\left(g\right)\)
\(n_{O2\left(pư\right)}=n_{SO2}=0,3\left(mol\right)\)
\(\Rightarrow n_{O2\left(dư\right)}=0,46875-0,3=0,16875\left(mol\right)\)
\(\Rightarrow m_{O2}=5,4\left(g\right)\)
Câu 3:
Ta có
\(n_{P2}=\frac{5,6.20\%}{22,4}=0,05\left(mol\right)\)
\(n_P=\frac{10}{31}=0,322\left(mol\right)\)
\(PTHH:4P+5O_2\rightarrow2P_2O_5\)
Ban đầu _0,322 ___0,05__
Phứng_0,04 ___0,05
Sau_ 0,282___0
Vậy Photpho dư
Câu 4:
\(C+O_2\rightarrow CO_2\)
x___________x
\(S+O_2\rightarrow SO_2\)
y________y
\(n_{SO2}=\frac{9,6}{32}=0,3\left(mol\right)\)
Giải hệ PT:
\(\left\{{}\begin{matrix}12x+32y=5,6\left(1\right)\\x+y=0,3\left(2\right)\end{matrix}\right.\)\(\Rightarrow\left\{{}\begin{matrix}x=0,2\\y=0,1\end{matrix}\right.\)
\(m_C=0,2.12=2,4\left(g\right)\)
\(m_S=m_{hh}-m_C=5,6-2,4=3,2\left(g\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%n_C=\frac{0,2}{0,2+0,1}.100\%=66,67\%\\\%n_S=100\%-66,67\%=33,33\%\end{matrix}\right.\)
cảm ơn bạn