Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{SO_2}=\dfrac{2,8}{22,4}=0,125mol\)
\(S+O_2\rightarrow\left(t^o\right)SO_2\)
0,125 0,125 ( mol )
\(2KMnO_4\rightarrow\left(t^o\right)K_2MnO_4+MnO_2+O_2\)
0,25 0,125 ( mol )
\(m_{KMnO_4}=0,25.158=39,5g\)

\(n_{KMnO_4}=\dfrac{m_{KMnO_4}}{M_{KMnO_4}}=\dfrac{47,4}{158}=0,3mol\)
\(n_{KMnO_4}=\dfrac{0,3}{80\%}=0,375mol\)
\(2KMnO_4+16HCl\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\)
2 16 2 2 5 8 ( mol )
0,375 > 2,5 ( mol )
0,375 0,9375 ( mol )
\(V_{Cl_2}=n_{Cl_2}.22,4=0,9375.22,4=21l\)
\(n_{KMnO_4\left(bd\right)}=\dfrac{47,4}{158}=0,3\left(mol\right)\) => \(n_{KMnO_4\left(pư\right)}=\dfrac{0,3.80}{100}=0,24\left(mol\right)\)
PTHH: 2KMnO4 + 16HCl --> 2KCl + 2MnCl2 + 5Cl2 + 8H2O
0,24------------------------------------->0,6
=> \(V=0,6.22,4=13,44\left(l\right)\)

$PTHH : 2KMnO_4 \xrightarrow[]{t^o} K_2MnO_4+MnO_2+O_2 \\ n_{O_2} = \dfrac{1,68}{22,4} = 0,075(mol) \\ n_{KMnO_4} = 2n_{O_2} = 0,15(mol) \\ m_{KMnO_4} = 0,15.158 = 23,7(gam) $
H% =( 23,7 : 31,6).100 = 75%

a)
nKMnO4 = 47.4/158 = 0.3 (mol)
2KMnO4 -to-> K2MnO4 + MnO2 + O2
0.3_________________________0.15
VO2 = 0.15*22.4 = 3.36 (l)
b)
nKMnO4 = 31.6/158 = 0.2 (mol)
2KMnO4 -to-> K2MnO4 + MnO2 + O2
0.2_________________________0.1
VO2 = 0.1*22.4 = 2.24 (l)
c)
nKMnO4 = 39.5/158 = 0.25 (mol)
2KMnO4 -to-> K2MnO4 + MnO2 + O2
0.25_________________________0.125
VO2 = 0.125*22.4 = 2.8 (l)
2)
a)
nO2 = 3.36/22.4 = 0.15 (mol)
2KMnO4 -to-> K2MnO4 + MnO2 + O2
0.3_________________________0.15
mKMnO4 = 0.3*158 = 47.4(g)
b)
nO2 = 8.96/22.4 = 0.4 (mol)
2KMnO4 -to-> K2MnO4 + MnO2 + O2
0.8_________________________0.4
mKMnO4 = 0.8*158 = 126.4(g)
c)
nO2 = 14.4/32 = 0.45 (mol)
2KMnO4 -to-> K2MnO4 + MnO2 + O2
0.9_________________________0.45
mKMnO4 = 0.9*158 = 142.2(g)

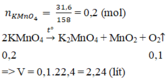

Đáp án B