Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


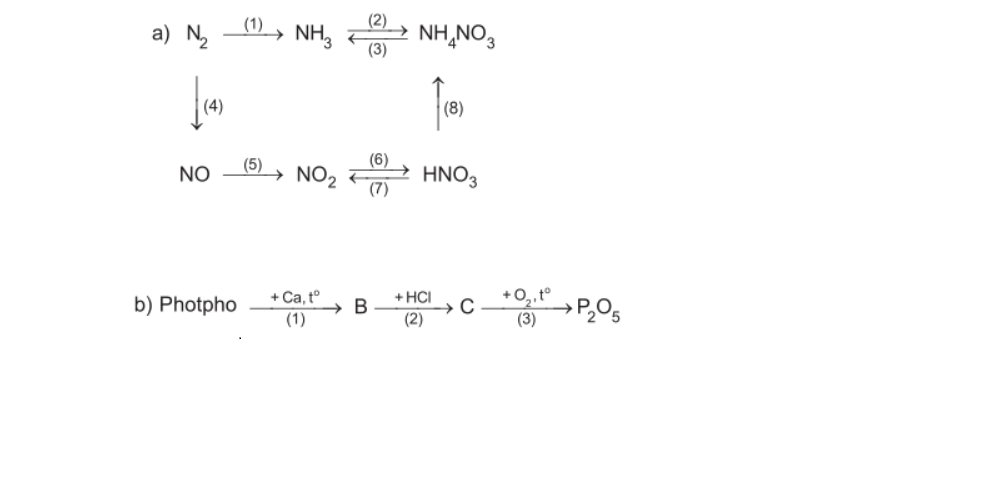
a)
\(\left(1\right)N_2+3H_2⇌\left(xt,t^o,P\right)2NH_3\\ \left(2\right)NH_3+HNO_3\rightarrow NH_4NO_3\\ \left(3\right)NH_4NO_3+KOH\rightarrow KNO_3+NH_3+H_2O\\ \left(4\right)N_2+O_2⇌\left(3000^oC\right)2NO\\ \left(5\right)2NO+O_2\rightarrow2NO_2\\ \left(6\right)4NO_2+O_2+2H_2O\rightarrow4HNO_3\\ \left(7\right)Cu+4HNO_{3\left(đ\right)}\underrightarrow{t^o}Cu\left(NO_3\right)_2+2NO_2+2H_2O\\ \left(8\right)NH_3+HNO_3\rightarrow NH_4NO_3\)
b)
\(\left(1\right)2P+3Ca\underrightarrow{to}Ca_3P_2\\ \left(2\right)Ca_3P_2+6HCl\rightarrow3CaCl_2+2PH_3\\ \left(3\right)2PH_3+4O_2\underrightarrow{to}P_2O_5+3H_2O\)
Lưu ý đối với các phản ứng 2 chiều, mình không có thêm được điều kiện trên mũi tên phản ứng (do đặc thù của latex hoc24.vn) vì thế mình có mở ngoặc sau, bạn nào sau này thấy thì trong ngoặc là đk phản ứng nhé!


CH3COONa(aq) + H2O(l) ⇌ CH3COOH(aq) + NaOH(aq)
Hiện tượng: Khi đun nhẹ bình (1), dung dịch trong bình (1) hóa hồng.
Nhận xét: Sau khi đun nhẹ, phản ứng thủy phân diễn ra tạo NaOH làm hoa hồng chỉ thị phenolphthalein.

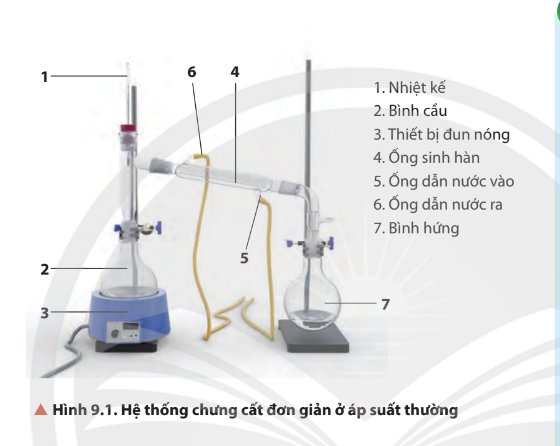
- Bước 2: để yên một thời gian, hỗn hợp trong phễu tách lớp. Tinh dầu quýt tan trong hexane, nước không ta trong hexane, do đó sau một thời gian sẽ có hiện tượng tách lớp: một lớp nước nặng hơn ở dưới và một lớp gồm hỗn hợp hexane, tinh dầu quýt nhẹ hơn ở trên.
- Bước 3: vặn khoá phễu từ từ, lớp nước phía dưới chảy vào bình tam giác, lớp trên là hỗn hợp hexane và tinh dầu quýt được lấy ra khỏi phễu bằng
- Bước 4: Làm bay hơi hexane để thu được tinh dầu quýt. Hexane có nhiệt độ sôi thấp hơn tinh dầu quýt, do đó khi làm bay hơi hỗn hợp, hexane bay hơi trước, còn lại tinh dầu quýt.

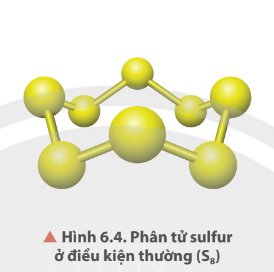
Ở dạng phân tử, sulfur gồm 8 nguyên tử liên kết cộng hoá trị với nhau tạo thành mạch vòng.

Khi đun nóng, phản ứng thủy phân diễn ra tạo NaOH làm hoa hồng chỉ thị phenolphthalein. Phản ứng chuyển dịch theo chiều thuận.

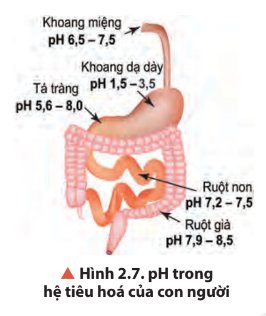
- Khoảng pH thấp nhất ở khoang dạ dày (pH 1,5 – 3,5).
- Khoảng pH cao nhất ở ruột già (pH 7,9 – 8,5).