
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


X: Fe3O4
Y: FeCl2
Z: FeCl3
T: Fe(OH)2
U: Fe(OH)3
A: NaCl (hoặc H2O)
B: H2O (hoặc NaCl)
D: H2 (hoặc Cl2)
E: Cl2 (hoặc H2)
F: NaOH
G: HCl
PTHH:
a) NaCl + H2O -dpmn----> 1/2 H2 + 1/2 Cl2 + NaOH
H2 + Cl2 -to-> 2 HCl
HCl + NaOH -> NaCl + H2O
b) 3 Fe +2 O2 -to->Fe3O4
Fe3O4 + 8 HCl -> FeCl2 +2 FeCl3 + H2O
FeCl2 + 2 NaOH -> Fe(OH)2 + 2 NaCl
FeCl3 +3 NaOH -> Fe(OH)3 + 3NaCl
Chúc em học tốt!

$2Fe + 6H_2SO_4 \to Fe_2(SO_4)_3 + 3SO_2 + 6H_2O(1)$
$Fe_2(SO_4)_3 + Fe\ to 3FeSO_4(2)$
Gọi $n_{Fe_2(SO_4)_3} = a(mol) ; n_{FeSO_4} = b(mol)$
Ta có : $400a + 152b = 62,8(1)$
$n_{SO_2} = 0,15(mol)$
$n_{Fe_2(SO_4)_3(1)} = \dfrac{1}{3}n_{SO_2} = 0,05(mol)$
$n_{Fe_2(SO_4)_3(2)} = \dfrac{1}{3}n_{FeSO_4} = \dfrac{b}{3}$
Suy ra:
$0,05 - \dfrac{b}{3} = a(2)$
Từ (1)(2) suy ra $a = \dfrac{45}{112} ; b = -1,055<0$
=> Sai đề


12.
Na2CO3+H2SO4->Na2SO4+H2O+CO2
............. 0,5 ............. ......... 0,5
CO2+2KOH->K2CO3+H2O
x 2x x
CO2+KOH->KHCO3
y y y
mKOH=98.40/100=39,2g
nKOH=39,2/56=0,7mol
Có:
2x+y=0,7
138x+100y=57,6
=>x=0,2mol; y=0,3mol
mK2CO3=138.0,2=27,6g
mKHCO3=57,6-27,6=30g
b.
nCO2=x+y=0,2+0,3=0,5mol
CMddH2SO4=0,5/0,2=2,5M
8. Hoàn thành sơ đồ chuyển hóa sau:
Mg \(\underrightarrow{\left(1\right)}\) MgO \(\underrightarrow{\left(2\right)}\) MgCl2 \(\underrightarrow{\left(3\right)}\) Mg(OH)2 \(\underrightarrow{\left(4\right)}\) MgO \(\underrightarrow{\left(5\right)}\) MgSO4 \(\underrightarrow{\left(6\right)}\) MgCO3 \(\underrightarrow{\left(7\right)}\) MgO
\(\left(1\right)2Mg+O_2\underrightarrow{t^o}2MgO\)
\(\left(2\right)MgO+2HCl\rightarrow MgCl_2+H_2O\)
\(\left(3\right)MgCl_2+2NaOH\rightarrow Mg\left(OH\right)_2\downarrow+2NaCl\)
\(\left(4\right)Mg\left(OH\right)_2\underrightarrow{t^o}MgO+H_2O\)
\(\left(5\right)MgO+H_2SO_4\rightarrow MgSO_4+H_2O\)
\(\left(6\right)MgSO_4+Na_2CO_3\rightarrow MgCO_3+Na_2SO_4\)
\(\left(7\right)MgCO_3\underrightarrow{t^o}MgO+CO_2\uparrow\)


Đặt CTHH của oxit sắt cần tìm : FexOy
PTHH : FexOy + yH2 = xFe + yH2O
0.2
Theo giả thiết C%H2SO4 còn 98% -3.405%= 94.595%
Hoặc \(\dfrac{98}{100+m_{H2O}}\) =0.94595
giải được mH2O=3.6g
nH2O=0.2 mol
Chất rắn thu được là Fe , nH2 thoát ra=3.36/22.4=0.15 mol
PTHH : Fe + H2SO4 --> FeSO4 + H2
0.15 0.15
Ta có tỉ lệ : nFe:nH2O = x:y = 0,15:0,2 = 3:4
Vậy CTHH của oxit sắt là Fe3O4

https://hoc24.vn/hoi-dap/question/71825.html bạn vào đây tham khảo nè




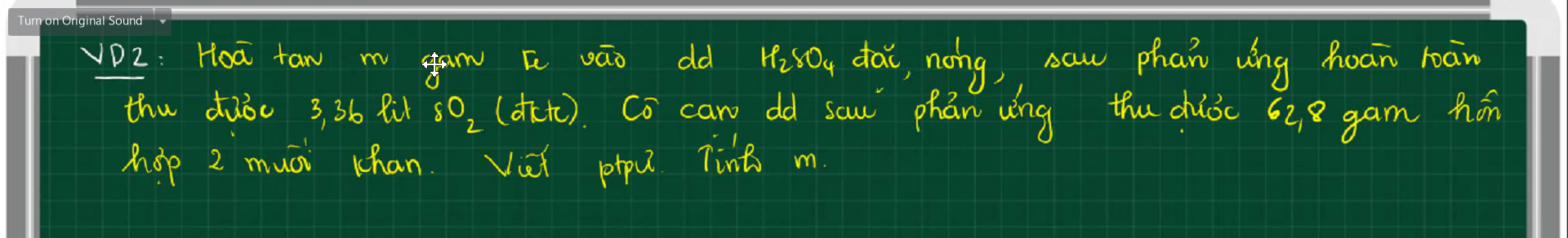


 mn giúp e những câu còn lại ạ :)
mn giúp e những câu còn lại ạ :)
a)
(1) \(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
(2) \(Fe+\dfrac{3}{2}Cl_2\xrightarrow[]{t^o}FeCl_3\)
(3) \(FeCl_2+\dfrac{1}{2}Cl_2\rightarrow FeCl_3\)
(4) \(2FeCl_3+Fe\rightarrow3FeCl_2\)
(5) \(FeCl_2+2KOH\rightarrow Fe\left(OH\right)_2+2KCl\)
(6) \(FeCl_3+3KOH\rightarrow Fe\left(OH\right)_3+3KCl\)
(7) \(Fe\left(OH\right)_2\xrightarrow[không.có.Oxi]{t^o}FeO+H_2O\)
(8) \(2Fe\left(OH\right)_3\xrightarrow[]{t^o}Fe_2O_3+3H_2O\)
(9) \(4Fe\left(OH\right)_2+O_2+2H_2O\rightarrow4Fe\left(OH\right)_3\)
(10) \(2FeO+\dfrac{1}{2}O_2\xrightarrow[]{t^o}Fe_2O_3\)
(11) \(Fe_2O_3+3H_2SO_4\rightarrow Fe_2\left(SO_4\right)_3+3H_2O\)
(12) \(Fe_2O_3+3CO\xrightarrow[]{t^o}2Fe+3CO_2\)
(13) \(2FeO+4H_2SO_{4\left(đ\right)}\xrightarrow[]{t^o}Fe_2\left(SO_4\right)_3+SO_2+4H_2O\)
(14) \(FeO+CO\xrightarrow[]{t^o}Fe+CO_2\)
c)
\(Ca+2H_2O\rightarrow Ca\left(OH\right)_2+H_2\uparrow\)
\(Ca\left(OH\right)_2+2HCl\rightarrow CaCl_2+2H_2O\)
\(CaCl_2+K_2CO_3\rightarrow2KCl+CaCO_3\)
\(CaCO_3+CO_2+H_2O\rightarrow Ca\left(HCO_3\right)_2\)
\(Ca\left(HCO_3\right)_2+2KOH\rightarrow CaCO_3+K_2CO_3+2H_2O\)
\(CaCO_3\xrightarrow[]{t^o}CaO+CO_2\)