Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Fe}=\dfrac{33.6}{56}=0.6\left(mol\right)\)
\(Fe_2O_3+3H_2\underrightarrow{^{^{t^0}}}2Fe+3H_2O\)
\(0.3..........0.9......0.6\)
\(m_{Fe_2O_3}=0.3\cdot160=48\left(g\right)\)
\(V_{H_2}=0.9\cdot22.4=20.16\left(l\right)\)

a, \(Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
b, \(n_{Fe}=\dfrac{22,4}{56}=0,4\left(mol\right)\)
Theo PT: \(n_{Fe_2O_3}=\dfrac{1}{2}n_{Fe}=0,2\left(mol\right)\Rightarrow m_{Fe_2O_3}=0,2.160=32\left(g\right)\)
c, \(n_{H_2}=\dfrac{3}{2}n_{Fe}=0,6\left(mol\right)\Rightarrow V_{H_2}=0,6.22,4=13,44\left(l\right)\)
d, \(2H_2+O_2\underrightarrow{t^o}2H_2O\)
Theo PT: \(n_{O_2}=\dfrac{1}{2}n_{H_2}=0,3\left(mol\right)\Rightarrow V_{O_2}=0,3.22,4=6,72\left(l\right)\)
\(\Rightarrow V_{kk}=\dfrac{V_{O_2}}{20\%}=33,6\left(l\right)\)
a)
$Fe_2O_3 + 3H_2 \xrightarrow{t^o} 2Fe + 3H_2O$
b) $n_{Fe} = \dfrac{22,4}{56} = 0,4(mol)$
Theo PTHH : $n_{Fe_2O_3} = \dfrac{1}{2}n_{Fe} = 0,2(mol)$
$m_{Fe_2O_3} = 0,2.160 = 32(gam)$
c) $n_{H_2} = \dfrac{3}{2}n_{Fe} = 0,6(mol)$
$V_{H_2} = 0,6.22,4 = 13,44(lít)$
d) $2H_2 + O_2 \xrightarrow{t^o} 2H_2O$
$V_{O_2} = \dfrac{1}{2}V_{H_2} = 6,72(lít)$
$V_{kk} = 6,72 : 20\% = 33,6(lít)$

PTHH: \(Fe_3O_4+4CO\xrightarrow[]{t^o}3Fe+4CO_2\uparrow\)
\(Fe+H_2SO_4\rightarrow FeSO_4+H_2\uparrow\)
\(H_2+\dfrac{1}{2}O_2\xrightarrow[]{t^o}H_2O\)
Ta có: \(n_{Fe_3O_4}=\dfrac{23,2}{232}=0,1\left(mol\right)\) \(\Rightarrow n_{Fe}=0,3\left(mol\right)=n_{H_2SO_4}=n_{H_2}=n_{H_2O}\)
\(\Rightarrow\left\{{}\begin{matrix}V_{H_2}=0,3\cdot22,4=6,72\left(l\right)\\V_{ddH_2SO_4}=\dfrac{0,3}{0,5}=0,6\left(l\right)\\V_{H_2O}=\dfrac{0,3\cdot18}{D_{nước}}=5,4\left(ml\right)\end{matrix}\right.\)
*P/s: \(D_{nước}=1g/ml\)

a) \(n_O=\dfrac{34,8-25,2}{16}=0,6\left(mol\right)\)
=> \(n_{H_2O}=0,6\left(mol\right)\) (bảo toàn O)
=> \(n_{H_2}=0,6\left(mol\right)\) (bảo toàn H)
=> \(V_{H_2}=0,6.22,4=13,44\left(l\right)\)
b) \(n_{Fe}=\dfrac{25,2}{56}=0,45\left(mol\right)\)
nFe : nO = 0,45 : 0,6 = 3 : 4
=> CTHH: Fe3O4
c) \(m_{H_2O}=0,6.18=10,8\left(g\right)\)
Mà \(d_{H_2O}=1\left(g/ml\right)\)
=> \(V_{H_2O}=10,8\left(ml\right)\)

\(n_{Mg}=\dfrac{6}{24}=0,25\left(mol\right)\\
pthh:Mg+H_2SO_4->MgSO_4+H_2\)
0,25 0,25 0,25 0,25
\(m_{MgSO_4}=0,25.120=30\left(g\right)\)
\(n_{Fe_2O_3}=\dfrac{24}{160}=0,15\left(mol\right)\\
pthh:Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
LTL : \(\dfrac{0,15}{1}>\dfrac{0,25}{3}\)
=> Fe dư , H2 hết
=> \(m_{Fe}=\dfrac{1}{6}.56=\approx9,3\left(g\right)\)
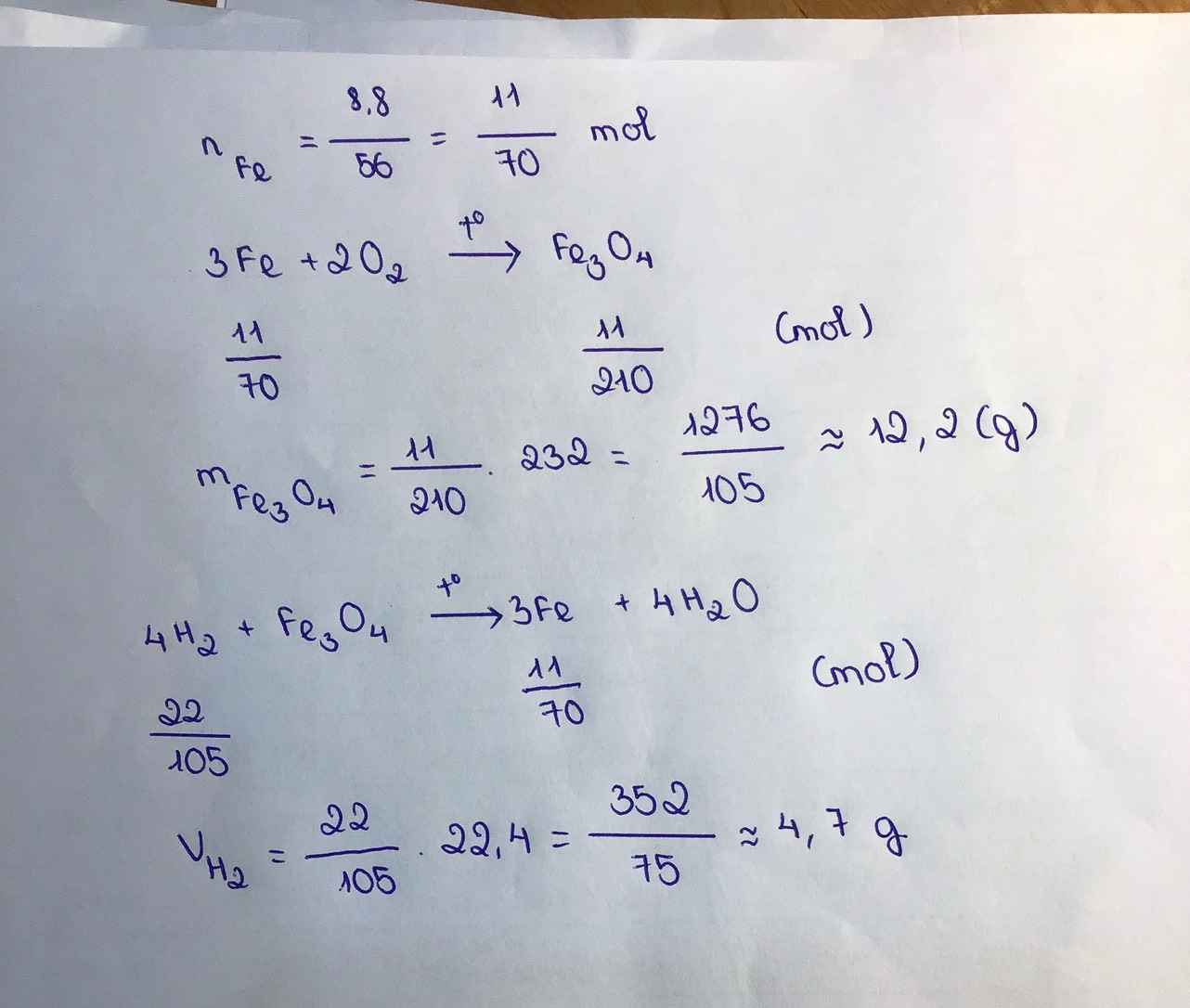

\(m_{Fe}=\dfrac{1,6}{160}=0,01\left(mol\right)\\ pthh:Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
0,01 0,03 0,02
=> \(V_{H_2}=0,03.22,4=0,672\left(l\right)\\ m_{Fe}=0,02.56=1,12\left(g\right)\)
\(n_{Fe_2O_3}=\dfrac{1,6}{160}=0,01\left(mol\right)\)
PTHH: Fe2O3 + 3H2 --to--> 2Fe + 3H2O
0,01---->0,03------->0,02
\(\rightarrow\left\{{}\begin{matrix}a,V_{H_2}=0,03.22,4=0,672\left(l\right)\\b,m_{Fe}=0,02.56=1,12\left(g\right)\end{matrix}\right.\)