Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(a)n_{H_2}=\dfrac{7,55}{22,4}=\dfrac{151}{448}mol\\ n_{Mg}=n_{Zn}=a;n_{Fe}=c\\ Mg+H_2SO_4\rightarrow MgSO_4+H_2\\ a.....a\\ Zn+H_2SO_4\rightarrow ZnSO_4+H_2\\ a.....a\\ Fe+H_2SO_4\rightarrow FeSO_4+H_2\\ b.....b\\ \Rightarrow\left\{{}\begin{matrix}24a+65a+56b=16\\2a+b=\dfrac{151}{448}\end{matrix}\right.\\ \Rightarrow a=0,125;b=\dfrac{39}{448}\\ \%m_{Mg}=\dfrac{24.0,125}{16}\cdot100=18,75\%\\ \%m_{Zn}=\dfrac{65.0,125}{16}\cdot100=50,78\%\\ \%m_{Fe}=100-18,75-50,78=30,47\%\\ b)V_{ddH_2SO_4}=\dfrac{0,125.2+\dfrac{39}{448}}{1}\approx0,337l\)

\(n_{H_2}=\dfrac{7,84}{22,4}=0,35\left(mol\right)\)
PT: \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
____0,35_____0,7___________0,35 (mol)
a, \(m_{Zn}=0,35.65=22,75\left(g\right)\)
b, \(C\%_{HCl}=\dfrac{0,7.36,5}{200}.100\%=12,775\%\)

Bài 4:
a) nH2= 6,72/22,4= 0,3(mol)
Đặt:nMg= x(mol); nZn=y(mol) (x,y>0)
PTHH: Mg + 2 HCl -> MgCl2 + H2
x_______2x________x_____x(mol)
Zn + 2 HCl -> ZnCl2 + H2
y____2y____y________y(mol)
Ta có hpt:
\(\left\{{}\begin{matrix}24x+65y=15,4\\x+y=0,3\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=0,1\\y=0,2\end{matrix}\right.\)
mMg=0,1.24=2,4(g)
=>%mMg = (2,4/15,4).100=15,584%
=>%mZn= 84,416%
b) nHCl(tổng)= 0,6(mol)
=> VddHCl=0,6/1=0,6(l)
Chúc em học tốt!

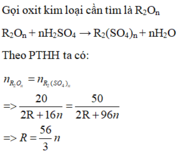
Vậy n = 3, R = 56 thỏa mãn, oxit là F e 2 O 3
n F e 2 O 3 = 20 160 = 0,125 m o l
m m u o i = m F e S O 4 + m F e 2 ( S O 4 ) 3
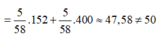
![]()
Đáp án: D

a) \(Zn + H_2SO_4 \rightarrow ZnSO_4 + H_2\)
Cu không pư H2SO4 loãng
b)
\(n_{H_2}=\dfrac{2,24}{22,4}= 0,1 mol\)
Theo PTHH:
\(n_{Zn}= n_{H_2}= 0,1 mol\)
\(\Rightarrow m_{Zn}= 0,1 . 65= 6,5 g\)
\(\Rightarrow m_{Cu}= m_{hh KL} - m_{Zn}= 10 - 6,5 = 3,5 g\)
Gọi \(n_{Cu}=x\left(mol\right)\)\(;n_{Zn}=y\left(mol\right)\)
\(n_{H_2}=\dfrac{2,24}{22,4}=0,1mol\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
0,1 0,1
\(m_{Zn}=0,1\cdot65=6,5g\)
\(m_{Cu}=10-6,4=3,6g\)

a, \(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\)
\(Cu+2H_2SO_{4\left(đ\right)}\underrightarrow{t^o}CuSO_4+SO_2+2H_2O\)
b, Ta có: \(n_{H_2}=\dfrac{2,479}{24,79}=0,1\left(mol\right)\)
\(n_{Zn}=n_{H_2}=0,1\left(mol\right)\)
\(n_{SO_2}=\dfrac{2,9748}{24,79}=0,12\left(mol\right)\)
\(n_{Cu}=n_{SO_2}=0,12\left(mol\right)\)
\(\Rightarrow m=m_{Zn}+m_{Cu}=0,1.65+0,12.64=14,18\left(g\right)\)
Có: \(n_{H_2SO_{4\left(đ\right)}}=2n_{SO_2}=0,24\left(mol\right)\Rightarrow x=m_{ddH_2SO_4\left(đ\right)}=\dfrac{0,24.98}{98\%}=24\left(g\right)\)
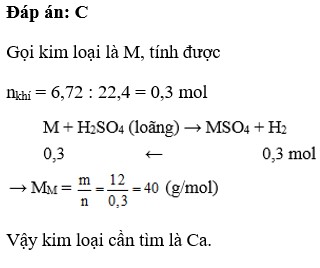

B.14
B đúng chứ