Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Trong dd 10%: mNaOH = 0,1.m1
Trong dd 40%: mNaOH = 0,4.m2
Trong 60 gam dd 20%: mNaOH = 12 gam
Có hệ: m dd = m1 + m2 = 60 gam
m NaOH = 0,1m1 + 0,4m2 = 12 gam
➝ m1 = 40 gam, m2 = 20 gam

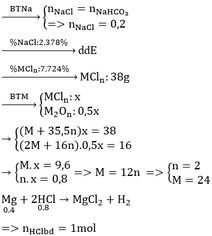
BTKL: mD + mNaHCO3 = mCO2 + mE
mD + 179,88 = 44.0,2 + 492 => mD = 320,92
BTKL: mMg + mddHCl = mH2 + mD
=> 24 . 0,4 + mddHCl = 2 . 0,4 + 320,92 => mddHCl = 312,12
=> C%HCl = 11,69%

a) 2Al (0,2) + 3H2SO4 (0,3) -----> Al2(SO4)3 + 3H2 (0,3)
b) - nH2 = 0,3 mol
- Theo PTHH: nAl = 0,2 mol
=> mAl = 5,4 gam
=> mCu = 4,6 gam
==>mhh=5,4+4,6=10 g
b Theo PTHH: nH2SO4 = 0,3 mol
=> mH2SO4 = 29,4 gam
=> mdd H2SO4 = 29,4.100\20=147gam
\(2Al+3H_2SO_4-->Al_2\left(SO_4\right)_3+3H_2\left(1\right)\)
0,2______0,3_____________0,1________0,3
\(n_{H_2}=\frac{6,72}{22,4}=0,3\left(mol\right)\)
a) => \(m=0,2.27+10=15,4\left(g\right)\)
b) \(m_{d^2H_2SO_4}=\frac{0,3.98.100}{20}=147\left(g\right)\)
c) \(Ba+2H_2O-->Ba\left(OH\right)_2+H_2\left(2\right)\)
0.35__________________0,35
\(n_{Ba}=\frac{47,95}{137}=0,35\left(mol\right)\)
\(3Ba\left(OH\right)_2+Al_2\left(SO_4\right)_3-->3BaSO_4\downarrow+2Al\left(OH\right)_3\downarrow\)
0,3___________0,1_______________0,3 _______0,2
\(2Al\left(OH\right)_3+Ba\left(OH\right)_2-->Ba\left(AlO_2\right)_2+4H_2O\left(3\right)\)
0,1__________0,05__________0,05
\(m_{d^2sau}=0,2.27+147-0,3.2+47,95-0,3.233-0,2.78=137,55\left(g\right)\)
\(C\%_{Ba\left(AlO_2\right)_2}=\frac{0,05.255}{137,55}.100=9,27\%\)

a) PTHH: \(SO_3+H_2O\rightarrow H_2SO_4\)
Ta có: \(n_{SO_3}=\dfrac{24}{80}=0,3\left(mol\right)=n_{H_2SO_4}\) \(\Rightarrow m_{ddH_2SO_4}=\dfrac{0,3\cdot98}{20\%}=147\left(g\right)\)
\(\Rightarrow V_{ddH_2SO_4}=\dfrac{147}{1,14}\approx128,95\left(ml\right)\)
b) PTHH: \(Fe+H_2SO_4\rightarrow FeSO_4+H_2\uparrow\)
Theo PTHH: \(n_{Fe}=n_{H_2SO_4}=n_{H_2}=0,3\left(mol\right)=n_{FeSO_4}\)
\(\Rightarrow\left\{{}\begin{matrix}m_{Fe}=0,3\cdot56=16,8\left(g\right)\\V_{H_2}=0,3\cdot24,76=7,428\left(l\right)\\m_{FeSO_4}=0,3\cdot152=45,6\left(g\right)\\m_{H_2}=0,3\cdot2=0,6\left(g\right)\end{matrix}\right.\)
Mặt khác: \(m_{dd}=m_{Fe}+m_{ddH_2SO_4}-m_{H_2}=163,2\left(g\right)\)
\(\Rightarrow C\%_{FeSO_4}=\dfrac{45,6}{163,2}\cdot100\%\approx27,94\%\)

a)
- Xét phần 1:
\(n_{CaCO_3}=\dfrac{10}{100}=0,1\left(mol\right)\)
PTHH: CaCO3 + 2CH3COOH --> (CH3COO)2Ca + CO2 + H2O
0,1----->0,2
=> \(n_{CH_3COOH\left(P1\right)}=0,2\left(mol\right)\)
- Xét phần 2:
\(n_{H_2}=\dfrac{0,224}{22,4}=0,01\left(mol\right)\)
PTHH: Mg + 2CH3COOH --> (CH3COO)2Mg + H2
0,02<---------------------0,01
=> \(n_{CH_3COOH\left(P2\right)}=0,02\left(mol\right)\)
=> \(n_{CH_3COOH\left(tổng\right)}=0,2+0,02=0,22\left(mol\right)\)
=> \(m_{CH_3COOH\left(tổng\right)}=60.0,22=13,2\left(g\right)\)
b)
\(n_{CH_3COOH\left(pư\right)}=\dfrac{0,22.75}{100}=0,165\left(mol\right)\)
PTHH: CH3COOH + C2H5OH --H2SO4(đ)to--> CH3COOC2H5 + H2O
0,165------------------------------>0,165
=> \(m_{CH_3COOC_2H_5}=0,165.88=14,52\left(g\right)\)
c)
\(n_{H_2}=\dfrac{34,944}{22,4}=1,56\left(mol\right)\)
PTHH: 2CH3COOH + 2Na --> 2CH3COONa + H2
0,22------------------------------->0,11
2H2O + 2Na --> 2NaOH + H2
2,9<------------------1,45
=> mdd = 13,2 + 2,9.18 = 65,4 (g)
=> \(V=\dfrac{65,4}{1,02}=\dfrac{1090}{17}\left(ml\right)\)
\(x=C_{M\left(A\right)}=\dfrac{0,22}{\dfrac{1,09}{17}}=\dfrac{374}{109}M\)

Ta có: \(n_{Fe_2O_3}=\dfrac{9,6}{160}=0,06\left(mol\right)\)
a. PTHH: Fe2O3 + 3H2SO4 ---> Fe2(SO4)3 + 3H2O (1)
Theo PT(1): \(n_{H_2SO_4}=3.n_{Fe_2O_3}=3.0,06=0,18\left(mol\right)\)
=> \(m_{H_2SO_4}=0,18.98=17,64\left(g\right)\)
Ta có: \(C_{\%_{H_2SO_4}}=\dfrac{17,64}{m_{dd_{H_2SO_4}}}.100\%=9,8\%\)
=> \(m_{dd_{H_2SO_4}}=180\left(g\right)\)
b. Ta có: \(m_{dd_{Fe_2\left(SO_4\right)_3}}=9,6+180=189,6\left(g\right)\)
Theo PT(1): \(n_{Fe_2\left(SO_4\right)_3}=n_{Fe_2O_3}=0,06\left(mol\right)\)
=> \(m_{Fe_2\left(SO_4\right)_3}=0,06.400=24\left(g\right)\)
=> \(C_{\%_{Fe_2\left(SO_4\right)_3}}=\dfrac{24}{189,6}.100\%=12,66\%\)
c. PTHH: Fe2(SO4)3 + 3BaCl2 ---> 3BaSO4↓ + 2FeCl3 (2)
Theo PT(2): \(n_{BaSO_4}=3.n_{Fe_2\left(SO_4\right)_3}=3.0,06=0,18\left(mol\right)\)
=> \(m_{BaSO_4}=0,18.233=41,94\left(g\right)\)
Theo PT(2): \(n_{BaCl_2}=n_{BaSO_4}=0,18\left(mol\right)\)
=> \(m_{BaCl_2}=0,18.208=37,44\left(g\right)\)
Ta có: \(C_{\%_{BaCl_2}}=\dfrac{37,44}{m_{dd_{BaCl_2}}}.100\%=10,4\%\)
=> \(m_{dd_{BaCl_2}}=360\left(g\right)\)
Chọn D