Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{H2}=\dfrac{11,155}{24,79}\approx0,45\left(mol\right)\)
a) Pt : \(Fe+H_2SO_4\rightarrow FeSO_4+H_2\uparrow\)
b) Theo Pt : \(n_{H2}=n_{Fe}=n_{H2SO4}=0,45\left(mol\right)\)
\(\Rightarrow m_{Fe}=0,45.56=25,2\left(g\right)\)
c) \(C_{MddH2SO4}=\dfrac{0,45}{0,6}=0,75\left(M\right)\)
Chúc bạn học tốt

a)
$n_{Al} = 0,3(mol)$
$2Al + 3H_2SO_4 \to Al_2(SO_4)_3 + 3H_2$
Theo PTHH :
$n_{H_2SO_4} = \dfrac{3}{2}n_{Al} = 0,45(mol)$
$m_{dd\ H_2SO_4} = \dfrac{0,45.98}{12,25\%} = 360(gam)$
b)
$n_{H_2} = n_{H_2SO_4} = 0,45(mol)$
$V_{H_2} = 0,45.22,4 = 10,08(lít)$
c)
$n_{Al_2(SO_4)_3} = 0,15(mol)$
$m_{dd\ sau\ pư} = 8,1 + 360 - 0,45.2 = 367,2(gam)$
$C\%_{Al_2(SO_4)_3} = \dfrac{0,15.342}{367,2}.100\% = 14\%$

PTHH: \(Fe+H_2SO_4\rightarrow FeSO_4+H_2\uparrow\)
\(H_2+\dfrac{1}{2}O_2\xrightarrow[]{t^o}H_2O\)
Ta có: \(n_{H_2}=\dfrac{0,896}{22,4}=0,04\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}n_{Fe}=0,04\left(mol\right)=n_{H_2SO_4}\\n_{O_2}=0,02\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{Fe}=0,04\cdot56=2,24\left(g\right)\\C_{M_{H_2SO_4}}=\dfrac{0,04}{0,5}=0,08\left(M\right)\\V_{O_2}=0,02\cdot22,4=0,448\left(l\right)\end{matrix}\right.\)

\(n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
PTHH:
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
0,2 0,6 0,2 0,3
\(m_{Al}=0,2.27=5,4\left(g\right)\)
\(C_{M\left(HCl\right)}=\dfrac{0,6}{0,6}=1\left(M\right)\)
\(m_{AlCl_3}=0,2.133,5=26,7\left(g\right)\)

\(n_{Al}=\dfrac{16,2}{27}=0,6\left(mol\right)\\ 2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\\ 0,6........0,9...........0,3........0,9\left(mol\right)\\ a.V_{H_2\left(đktc\right)}=0,9.22,4=20,16\left(l\right)\\ b.C_{MddH_2SO_4}=\dfrac{0,9}{0,5}=1,8\left(M\right)\\ c.C_{MddX}=C_{MddAl_2\left(SO_4\right)_3}=\dfrac{0,3}{0,5}=0,6\left(M\right)\)

\(nHCl=0,2.0,3=0,06\\ 2Al+6HCl=>2AlCl3+3H2\\ =>nAl=0,02\left(mol\right)\\ =>mAl=0,02.27=0,54\left(g\right)\\ tacónAlCl3=0,02\left(mol\right)\\ =>Cm\left(AlCl3\right)=\dfrac{0,02}{0,2}=0,1\left(M\right)\)
a, \(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
b, \(n_{HCl}=0,2.0,3=0,06\left(mol\right)\)
Theo PT: \(n_{Al}=\dfrac{1}{3}n_{HCl}=0,02\left(mol\right)\)
\(\Rightarrow m_{Al}=0,02.27=0,54\left(g\right)\)
c, \(n_{AlCl_3}=\dfrac{1}{3}n_{HCl}=0,02\left(mol\right)\)
\(\Rightarrow C_{M_{AlCl_3}}=\dfrac{0,02}{0,2}=0,1\left(M\right)\)
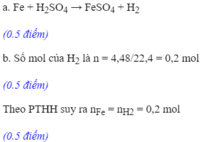

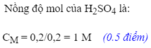
a) Fe + H2SO4 ----> FeSO4 + H2
nH2 = 0,45 mol
- theo pthh: nFe = 0,45 mol
=> mFe = 25,2 gam
b) - theo pthh: nH2SO4 = 0,45 mol
=> CM H2SO4 = 0,75M
c) - theo pthh: nFeSO4 = 0,45 mol
=> mFeSO4 = 68,4 gam
nH2 = \(\dfrac{V}{22,4}\)=\(\dfrac{10,08}{22,4}\)= 0,45 (mol)
PTHH:
Fe + H2SO4 → FeSO4 + H2
1 : 1 : 1 : 1 (mol)
0,45 : 0,45 : 0,45 : 0,45 (mol)
a) mFe = n.M = 0,45. 56 = 25,2 (g)
b) CM dd H2SO4 = \(\dfrac{n}{V}\)=\(\dfrac{0,45}{0,6}\)= 0,75 (M)
c) mFeSO4 = n.M = 0,45.152 = 68,4 (g)