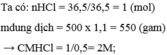Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Ta có: \(m_{ddCuSO_4}=\dfrac{3}{15\%}=20\left(g\right)\)
\(V_{ddCuSO_4}=\dfrac{20}{1,15}\approx17,39\left(ml\right)\)
Ta có: \(n_{CuSO_4}=\dfrac{3}{160}=0,01875\left(mol\right)\)
\(\Rightarrow C_{M_{CuSO_4}}=\dfrac{0,01875}{0,01739}\approx1,08M\)
Bạn tham khảo nhé!

1)
\(m_{ddCuSO_4\left(bd\right)}=1,6.25=40\left(g\right)\)
\(n_{CuSO_4.5H_2O}=\dfrac{11,25}{250}=0,045\left(mol\right)\)
=> \(n_{CuSO_4}=0,045\left(mol\right)\)
\(C_M=\dfrac{0,045}{0,025}=1,8M\)
\(C\%=\dfrac{0,045.160}{40}.100\%=18\%\)
b)
\(m_{CuSO_4}=\dfrac{200.18}{100}=36\left(g\right)\)
\(n_{CuSO_4.5H_2O}=\dfrac{5,634}{250}=0,022536\left(mol\right)\)
nCuSO4 (tách ra) = 0,022536 (mol)
=> \(m_{CuSO_4\left(dd.ở.t^o\right)}=36-0,022536.160=32,39424\left(g\right)\)
\(m_{H_2O\left(bd\right)}=200-36=164\left(g\right)\)
nH2O (tách ra) = 0,022536.5 = 0,11268 (mol)
=> \(m_{H_2O\left(dd.ở.t^o\right)}=164-0,11268.18=161,97176\left(g\right)\)
\(S_{t^oC}=\dfrac{32,39424}{161,97176}.100=20\left(g\right)\)

1/ \(n_A=\dfrac{m_{dd}.C\%}{100.M_A}\)
2/ \(V_{dd}=\dfrac{m}{D}\)
=> \(C_M=\dfrac{n}{V}=\dfrac{n.D}{m}\)

\(n_{Na_2CO_3}=n_{Na_2CO_3\cdot10H_2O}=\dfrac{57.2}{106+18\cdot10}=0.2\left(mol\right)\)
\(C_{M_{Na_2CO_3}}=\dfrac{0.2}{0.4}=0.5\left(M\right)\)
\(m_{Na_2CO_3}=0.2\cdot106=21.2\left(g\right)\)
\(m_{dd}=400\cdot1.05=420\left(g\right)\)
\(C\%_{Na_2CO_3}=\dfrac{21.2}{420}\cdot100\%=5.04\%\)

\(n_{H_2SO_4}=0.1\cdot2=0.2\left(mol\right)\)
\(m_{dd_{H_2SO_4}}=100\cdot1.2=120\left(g\right)\)
\(n_{BaCl_2}=0.1\cdot1=0.1\left(mol\right)\)
\(m_{dd_{BaCl_2}}=100\cdot1.32=132\left(g\right)\)
\(BaCl_2+H_2SO_4\rightarrow BaSO_4+2HCl\)
\(0.1................0.1.........0.1...............0.2\)
\(\Rightarrow H_2SO_4dư\)
\(m_{BaSO_4}=0.1\cdot233=23.3\left(g\right)\)
\(V_{dd}=0.1+0.1=0.2\left(l\right)\)
\(C_{M_{H_2SO_4\left(dư\right)}}=\dfrac{0.2-0.1}{0.2}=0.5\left(M\right)\)
\(C_{M_{HCl}}=\dfrac{0.2}{0.2}=1\left(M\right)\)
\(m_{\text{dung dịch sau phản ứng}}=120+132-23.3=228.7\left(g\right)\)
\(C\%_{H_2SO_4\left(dư\right)}=\dfrac{0.1\cdot98}{228.7}\cdot100\%=4.28\%\)
\(C\%_{HCl}=\dfrac{0.2\cdot36.5}{228.7}\cdot100\%=3.2\%\)

\(C\%=\dfrac{30}{170}.100\%=17,647\%\)
\(V_{\text{dd}}=\left(30+170\right)1,1=220ml\)
\(n_{NaCl}=\dfrac{30}{58,5}=0,513mol\)
\(C_M=\dfrac{0,513}{0,22}=0,696M\)
\(C\%_{NaCl}=\dfrac{30}{170+30}.100\%=15\%\\ C_M=C\%.\dfrac{10D}{M}=10.\dfrac{10.1,1}{58,5}=1,88M\)

\(n_{Na_2CO_3}=\dfrac{10,6}{106}=0,1\left(mol\right)\\ \rightarrow C_{M\left(Na_2CO_3\right)}=\dfrac{0,1}{0,2}=0,5M\)
Ta có: \(C\%=\dfrac{C_M.M}{10.D}\)
\(\rightarrow C\%=\dfrac{0,5.106}{10.1,05}=5,05\%\)

\(n_{P_2O_5}=\dfrac{99,4}{142}=0,7\left(mol\right)\)
\(P_2O_5+3H_2O\rightarrow2H_3PO_4\)
0,7 2,1 1,4
a, \(m_{H_3PO_4}=1,4.98=137,2\left(g\right)\)
\(m_{ddH_3PO_4}=99,4+500=599,4\left(g\right)\)
Kl nước trong dd A :
\(m_{H_2O}=599,4-137,2=462,2\left(g\right)\)
\(b,C\%_{H_3PO_4}=\dfrac{137,2}{599,4}.100\%\approx22,89\%\)
\(c,C_M=\dfrac{n}{V}=\dfrac{1,4}{0,5}=2,8M\)