
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(a.\)
\(n_C=\dfrac{9.6}{12}=0.8\left(mol\right)\)
\(n_{O_2}=\dfrac{1.12}{22.4}=0.05\left(mol\right)\)
\(C+O_2\underrightarrow{^{t^0}}CO_2\)
\(0.05...0.05..0.05\)
\(\Rightarrow Cdư\)
\(V_{CO_2}=0.05\cdot22.4=1.12\left(l\right)\)
\(b.\)
\(n_P=\dfrac{6.2}{31}=0.2\left(mol\right)\)
\(n_{O_2}=\dfrac{1.12}{22.4}=0.05\left(mol\right)\)
\(4P+5O_2\underrightarrow{^{t^0}}2P_2O_5\)
\(0.04....0.05......0.02\)
\(\Rightarrow Pdư\)
\(m_{P_2O_5}=0.02\cdot142=2.84\left(g\right)\)

\(a,PTHH:2Al+6HCl\to 2AlCl_3+3H_2\\ b,n_{Al}=\dfrac{5,4}{27}=0,2(mol)\\ \Rightarrow n_{H_2}=\dfrac{3}{2}n_{Al}=0,3(mol)\\ \Rightarrow V_{H_2}=0,3.22,4=6,72(l)\\ c,n_{AlCl_3}=n_{Al}=0,2(mol)\\ \Rightarrow m_{AlCl_3}=0,2.133,5=26,7(g)\)



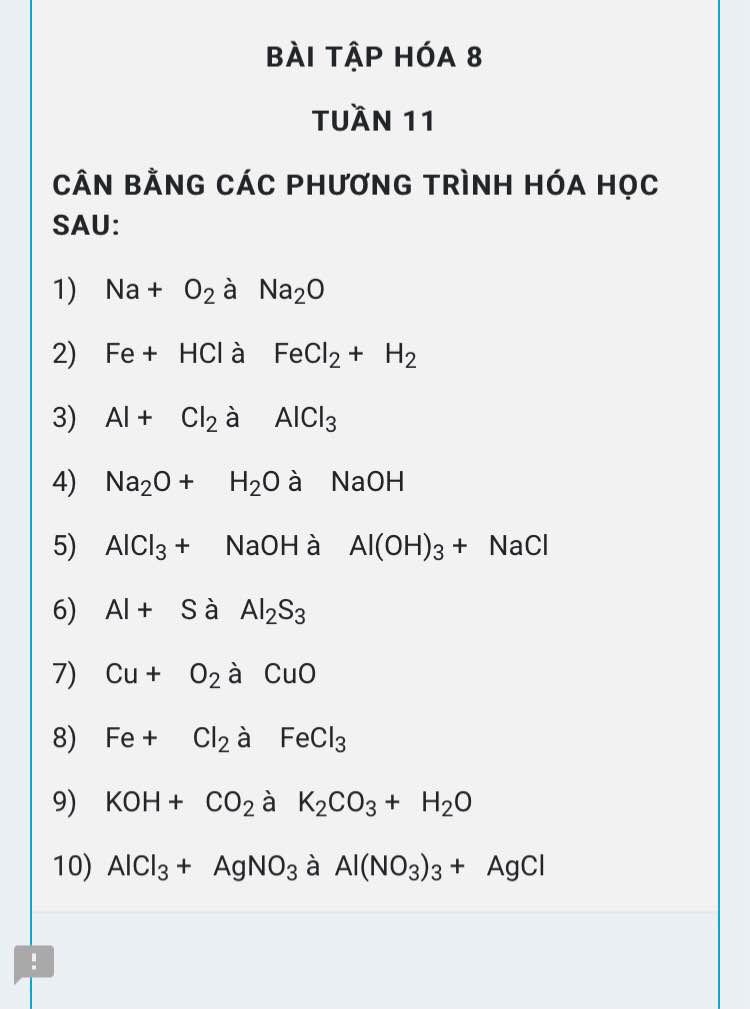



 mn giải giúp em với ạ em đag cần gấp , em cảm ơn
mn giải giúp em với ạ em đag cần gấp , em cảm ơn




\(4Na+O_2\rightarrow2Na_2O\\ Fe+2HCl\rightarrow FeCl_2+H_2\\ 2Al+3Cl_2\underrightarrow{^{to}}2AlCl_3\\ Na_2O+H_2O\rightarrow2NaOH\\ AlCl_3+3NaOH\rightarrow Al\left(OH\right)_3+3NaCl\\ 2Al+3S\underrightarrow{^{to}}Al_2S_3\\ 2Cu+O_2\underrightarrow{^{to}}2CuO\\ 2Fe+3Cl_2\underrightarrow{^{to}}2FeCl_3\\ 2KOH+CO_2\rightarrow K_2CO_3+H_2O\\ AlCl_3+3AgNO_3\rightarrow Al\left(NO_3\right)_3+3AgCl\)