Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

BTKL : \(m_{CO_2}+m_{H_2O}=m_X+m_{O_2}=15,96\left(g\right)\)
=> \(m_{binhtang}=m_{CO_2}+m_{H_2O}=15,96\left(g\right)\)

\(n_{N_2\left(tổng\right)}=\dfrac{4,816}{22,4}=0,215\left(mol\right)\)
\(n_{CaCO_3}=\dfrac{3}{100}=0,03\left(mol\right)\)
=> nCO2 = 0,03 (mol)
=> \(n_{C_xH_yN}=\dfrac{0,03}{x}\left(mol\right)\)
=> \(M_{C_xH_yN}=\dfrac{0,59}{\dfrac{0,03}{y}}=\dfrac{59}{3}x\left(mol\right)\)
=> 12x + y + 14 = \(\dfrac{59}{3}x\)
=> \(\dfrac{-23}{3}x+y=-14\) (1)
Bảo toàn H: \(n_{H_2O}=\dfrac{0,03y}{2x}\left(mol\right)\)
Bảo toàn N: \(n_{N_2\left(kk\right)}=\dfrac{0,215.2-\dfrac{0,03}{x}}{2}=0,215-\dfrac{0,015}{x}\left(mol\right)\)
Mà nN2 = 4.nO2
=> \(n_{O_2}=0,05375-\dfrac{0,00375}{x}\left(mol\right)\)
Bảo toàn O: \(0,1075-\dfrac{0,0075}{x}=0,06+\dfrac{0,03y}{2x}\)
=> \(0,03y+0,015=0,095x\) (2)
(1)(2) => x = 3; y = 9
CTPT: C3H9N

nCH4 = 11.2/22.4 = 0.5 (mol)
CH4 + 2O2 -to-> CO2 + 2H2O
0.5____________0.5
CO2 + Ca(OH)2 => CaCO3 + H2O
0.5_______________0.5
mCaCO3 = 0.5*100 = 50 (g)

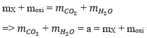

\(n_{CaCO_3}=\dfrac{7,5}{100}=0,075\left(mol\right)\)
=> nC = 0,075 (mol)
Có \(n_{CO_2}=n_C=0,075\left(mol\right)\)
=> \(n_{H_2O}=\dfrac{4,2-0,075.44}{18}=0,05\left(mol\right)\)
=> nH = 0,1 (mol)
\(n_{O_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
Bảo toàn O: \(n_{O\left(A\right)}=0,075.2+0,05-0,1.2=0\left(mol\right)\)
=> A chứa C, H
mA = mC + mH = 0,075.12 + 0,1.1 = 1 (g)
\(m_{tăng}=m_{H_2O}+m_{CO_2}=4,2\left(g\right)\\ n_{CaCO_3}=\dfrac{7,5}{100}=0,075\left(mol\right)\)
PTHH: Ca(OH)2 + CO2 ---> CaCO3 + H2O
0,075 0,075
\(\rightarrow m_{CO_2}=0,075.44=3,3\left(g\right)\\ \rightarrow m_{H_2O}=4,2-3,3=0,9\left(g\right)\\ \rightarrow n_{H_2O}=\dfrac{0,9}{18}=0,05\left(mol\right)\\ \rightarrow n_{O\left(sau.pư\right)}=0,05+0,075.2=0,1\left(mol\right)\\ n_{O\left(trong.O_2\right)}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\\ \rightarrow\left\{{}\begin{matrix}n_C=0,075\left(mol\right)\\n_H=0,05.2=0,1\left(mol\right)\\n_O=0,1-0,1=0\left(mol\right)\end{matrix}\right.\)
=> mA = 0,075.12 + 0,1.1 + 0 = 1 (g)