Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

nFeS2 = 120/120 = 1 (mol)
PTHH: 4FeS2 + 11O2 -> (t°) 2Fe2O3 + 8SO2
Mol: 1 ---> 2,75 ---> 0,5 ---> 2
VO2 = 2,75/(100% - 10%) . 22,4 = 616/9 (l)
msp = (0,5 . 160 + 8 . 64) . 80% = 437,6 (g)

nFeS2 = 120/120 = 1 (mol)
PTHH: 4FeS2 + 11O2 -> (t°) 2Fe2O3 + 8SO2
Mol: 1 ---> 2,75 ---> 0,5 ---> 2
VO2 = 2,75/(100% - 10%) . 22,4 = 616/9 (l)
msp = (0,5 . 160 + 8 . 64) . 80% = 437,6 (g)

\(n_{C_2H_2}=\dfrac{2,6}{26}=0,1\left(mol\right)\\ 2C_2H_2+5O_2\rightarrow\left(t^o\right)4CO_2+2H_2O\\ a,n_{O_2}=\dfrac{5}{2}.n_{C_2H_2}=\dfrac{5}{2}.0,1=0,25\left(mol\right)\\ \Rightarrow V_{O_2\left(đktc\right)}=0,25.22,4=5,6\left(l\right)\\ b,n_{CO_2}=\dfrac{4}{2}.n_{C_2H_2}=\dfrac{4}{2}.0,1=0,2\left(mol\right)\\ \Rightarrow m_{CO_2}=0.2.44=8,8\left(g\right)\\ n_{H_2O}=n_{C_2H_2}=0,1\left(mol\right)\\ \Rightarrow m_{H_2O}=0,1.18=1,8\left(g\right)\\ \Rightarrow m_{sp}=m_{CO_2}+m_{H_2O}=8,8+1,8=10,6\left(g\right)\)

\(a,n_{FeS_2}=\dfrac{m_{FeS_2}}{M_{FeS_2}}=\dfrac{6}{120}=0,05\left(mol\right)\\ 4FeS_2+11O_2\rightarrow\left(t^o,xt\right)2Fe_2O_3+8SO_2\uparrow\\ n_{Fe_2O_3}=\dfrac{2}{4}.n_{FeS_2}=\dfrac{2}{4}.0,05=0,025\left(mol\right)\\ \Rightarrow m_{Fe_2O_3}=160.0,025=4\left(g\right)\\ n_{SO_2}=\dfrac{8}{4}.n_{FeS_2}=\dfrac{8}{4}.0,05=0,1\left(mol\right)\\ \Rightarrow m_{SO_2}=0,1.64=6,4\left(g\right)\\ \Rightarrow m_{sp}=m_{Fe_2O_3}+m_{SO_2}=4+6,4=10,4\left(g\right)\\ b,n_{O_2}=\dfrac{11}{4}.n_{FeS_2}=\dfrac{11}{4}.0,05=0,1375\left(mol\right)\\ \Rightarrow V_{O_2\left(đktc\right)}=0,1375.22,4=3,08\left(l\right)\\ \Rightarrow V_{kk\left(đktc\right)}=3,08.5=15,4\left(l\right)\)
\(pthh:4FeS_2+11O_2\overset{t^o}{--->}2Fe_2O_3+8SO_2\uparrow\)
a. Ta có: \(n_{FeS_2}=\dfrac{6}{120}=0,05\left(mol\right)\)
Theo pt: \(n_{O_2}=\dfrac{11}{4}.n_{FeS_2}=\dfrac{11}{4}.0,05=0,1375\left(mol\right)\)
\(\Rightarrow m_{sản.phẩm.thu.được}=6+0,1375.32=10,4\left(g\right)\)
b. Ta có: \(V_{O_2}=0,1375.22,4=3,08\left(lít\right)\)
Mà: \(V_{O_2}=\dfrac{1}{5}V_{kk}\)
\(\Rightarrow V_{kk}=3,08.5=15,4\left(lít\right)\)

a, \(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
b, \(n_{O_2}=\dfrac{7,437}{24,79}=0,3\left(mol\right)\)
Theo PT: \(n_{Al_2O_3}=\dfrac{2}{3}n_{O_2}=0,2\left(mol\right)\)
\(\Rightarrow m_{Al_2O_3}=0,2.102=20,4\left(g\right)\)
c, \(H=\dfrac{18,36}{20,4}.100\%=90\%\)

\(n_{KClO_3}=\dfrac{24,5}{122,5}=0,2mol\)
\(2KClO_3\rightarrow\left(t^o,MnO_2\right)2KCl+3O_2\)
0,2 0,2 0,3 ( mol )
\(V_{O_2}=0,3.24,79=7,437l\)
\(m_{KCl}=0,2.74,5=14,9g\)

\(a.2H_2+O_2-^{t^o}\rightarrow2H_2O\\ b.n_{H_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\\ n_{O_2}=\dfrac{1}{2}n_{H_2}=0,2\left(mol\right)\\ \Rightarrow V_{O_2}=0,2.22,4=4,48\left(l\right)\\ TrongkhôngkhíO_2chiếm20\%\\ \Rightarrow V_{kk}=\dfrac{4,48}{20\%}=22,4\left(l\right)\)

\(n_P=\dfrac{6.2}{31}=0.2\left(mol\right)\)
\(n_{O_2}=\dfrac{8.96}{22.4}=0.4\left(mol\right)\)
\(4P+5O_2\underrightarrow{^{^{t^0}}}2P_2O_5\)
\(4........5\)
\(0.2.........0.4\)
Lập tỉ lệ : \(\dfrac{0.2}{4}< \dfrac{0.4}{5}\Rightarrow O_2dư\)
\(n_{P_2O_5}=0.2\cdot\dfrac{2}{4}=0.1\left(mol\right)\)
\(m_{P_2O_5}=0.1\cdot142=14.2\left(g\right)\)
\(m_{P_2O_5\left(tt\right)}=14.2\cdot80\%=11.36\left(g\right)\)

a) \(n_{C_4H_{10}}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
PTHH: 2C4H10 + 13O2 --to--> 8CO2 + 10H2O
0,4---->2,6---------->1,6------->2
=> m = 1,6.44 = 70,4 (g)
b) \(V_{O_2}=2,6.22,4=58,24\left(l\right)\)
c) \(n_P=\dfrac{9,1}{31}=\dfrac{91}{310}\left(mol\right)\)
PTHH: 4P + 5O2 --to--> 2P2O5
Xét tỉ lê \(\dfrac{\dfrac{91}{310}}{4}< \dfrac{2,6}{5}\) => P hết, O2 dư
\(m_{P_2O_5}=\dfrac{91}{620}.142=\dfrac{6461}{310}\left(g\right)\)
Tên sản phẩm: Điphotpho pentaoxit
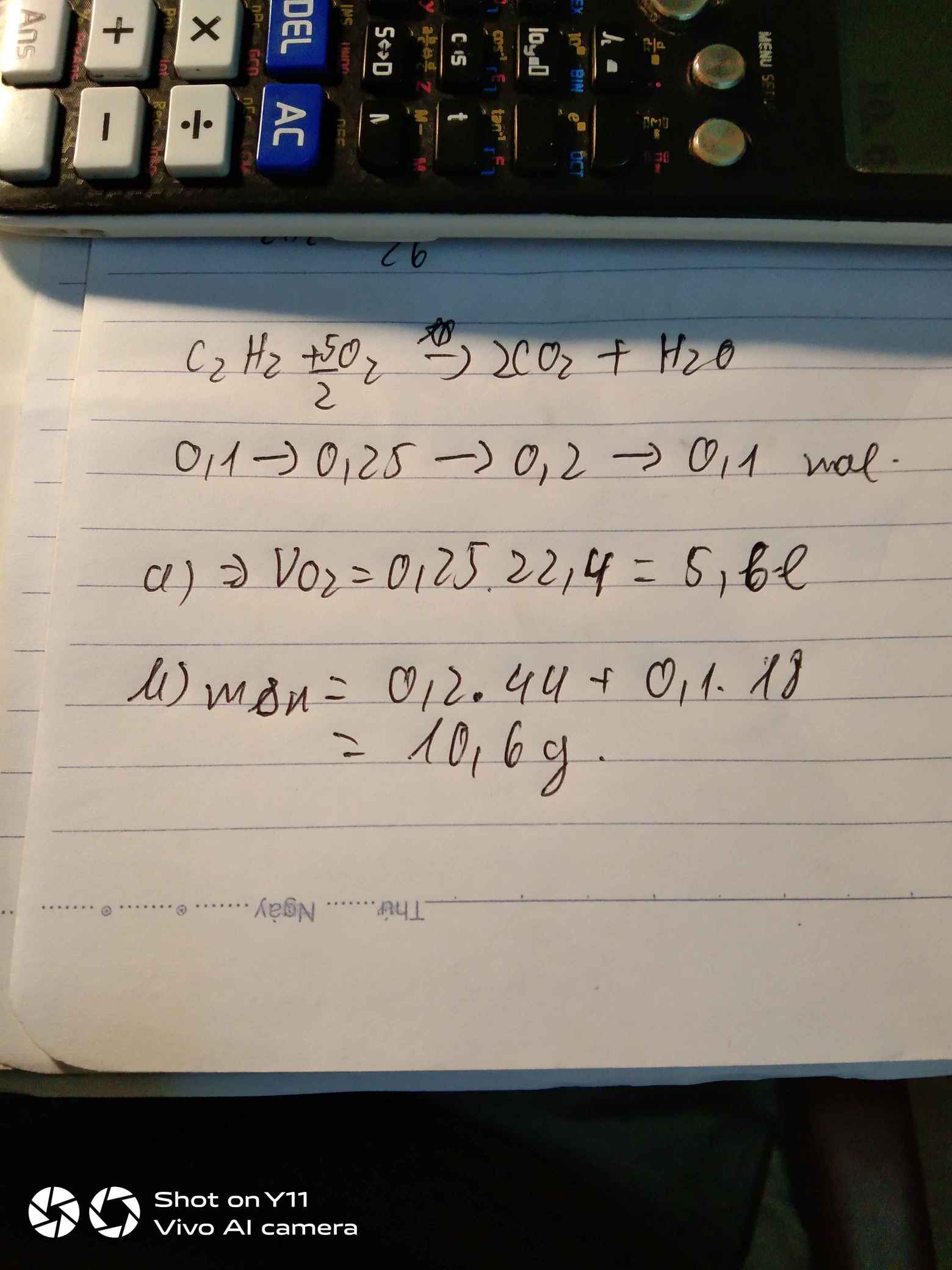
4FeS2+11O2-to>2Fe2O3+8SO2
1-------------2,75-------0,5-------2 mol
n FeS2=\(\dfrac{120}{120}=1mol\)
=>VO2=2,75.\(\dfrac{110}{100}\).32=96,8g
H=80%
=>m Fe2O3=0,5.160.\(\dfrac{80}{100}\)=64g
Có 100% mà hụt 10% thì sẽ là 100% - 10% = 90% nhé chị:)