Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

A tác dụng với NaHCO3 cho khí CO2 → A: axit CH3COOH
BTKL: m + mO2 = mCO2 + mH2O => m = 1,8
=> nCH3COOH = 0,03
CH3COOH + C2H5OH → CH3COOC2H5 + H2O
0,03 0,02 0,0125
=> H = 62,5%

a, \(C_2H_6O+3O_2\underrightarrow{t^o}2CO_2+3H_2O\)
b, \(n_{CO_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
Theo PT: \(n_{O_2}=\dfrac{3}{2}n_{CO_2}=0,3\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,3.22,4=6,72\left(l\right)\)
c, \(n_{C_2H_6O}=\dfrac{1}{2}n_{CO_2}=0,1\left(mol\right)\)
\(\Rightarrow m_{C_2H_6O}=0,1.46=4,6\left(g\right)\)
\(\Rightarrow V_{C_2H_6O}=\dfrac{4,6}{0,8}=5,75\left(ml\right)\)
Độ rượu = \(\dfrac{5,75}{50}.100=11,5^o\)

a, nC2H4 = 2,479/24,79 = 0,1 (mol)
PTHH: C2H4 + 3O2 -> (t°) 2CO2 + 2H2O
Mol: 0,1 ---> 0,3
VO2 = 0,3 . 24,79 = 7,437 (l)
b, PTHH: 2C2H2 + 5O2 -> (t°) 4CO2 + 2H2O
Mol: 0,12 <--- 0,3
VC2H2 = 0,12 . 24,79 = 2,9748 (l)

\(n_{C_2H_5OH}=\dfrac{23}{46}=0,5\left(mol\right)\\a, PTHH:C_2H_5OH+3O_2\rightarrow\left(t^o\right)2CO_2+3H_2O\\ b,n_{O_2}=3.0,5=1,5\left(mol\right)\\ V_{O_2\left(đktc\right)}=22,4.1,5=33,6\left(l\right)\\ c,V_{C_2H_5OH}=46\%.100=46\left(ml\right)\\ C_2H_5OH+Na\rightarrow C_2H_5ONa+\dfrac{1}{2}H_2\\ n_{C_2H_5OH}=\dfrac{0,8.46}{46}=0,8\left(mol\right)\\ n_{H_2}=\dfrac{0,8}{2}=0,4\left(mol\right)\Rightarrow V_{H_2\left(đktc\right)}=0,4.22,4=8,96\left(l\right)\)

\(n_{CO2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
\(C_2H_5OH+3O_2\xrightarrow[]{t^o}2CO_2+3H_2O\)
0,1 0,3 0,2
b) \(m_{C2H5OH}=0,1.46=4,6\left(g\right)\)
c) \(V_{O2\left(dktc\right)}=0,3.22,4=6,72\left(l\right)\)
Chúc bạn học tốt

1) \(n_{C_2H_4}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
PTHH: C2H4 + H2O --axit--> C2H5OH
0,4-------------------->0,4
=> mC2H5OH = 0,4.46.70% = 12,88 (g)
\(V_{C_2H_5OH}=\dfrac{12,88}{0,8}=16,1\left(ml\right)\\ \rightarrowĐ_r=\dfrac{16,1}{50}.100=32,2^o\)
2) \(\left\{{}\begin{matrix}n_{C_2H_5OH}=\dfrac{36,8}{46}=0,8\left(mol\right)\\n_{CH_3COOH}=\dfrac{36}{60}=0,6\left(mol\right)\\n_{CH_3COOC_2H_5}=\dfrac{44}{88}=0,5\left(mol\right)\end{matrix}\right.\)
PTHH: CH3COOH + C2H5OH --H2SO4(đặc), to--> CH3COOC2H5 + H2O
0,5<-------------------------------------------------0,5
LTL: 0,6 < 0,8 => Hiệu suất phản ứng tính theo CH3COOH
=> \(H=\dfrac{0,5}{0,6}.100\%=83,33\%\)
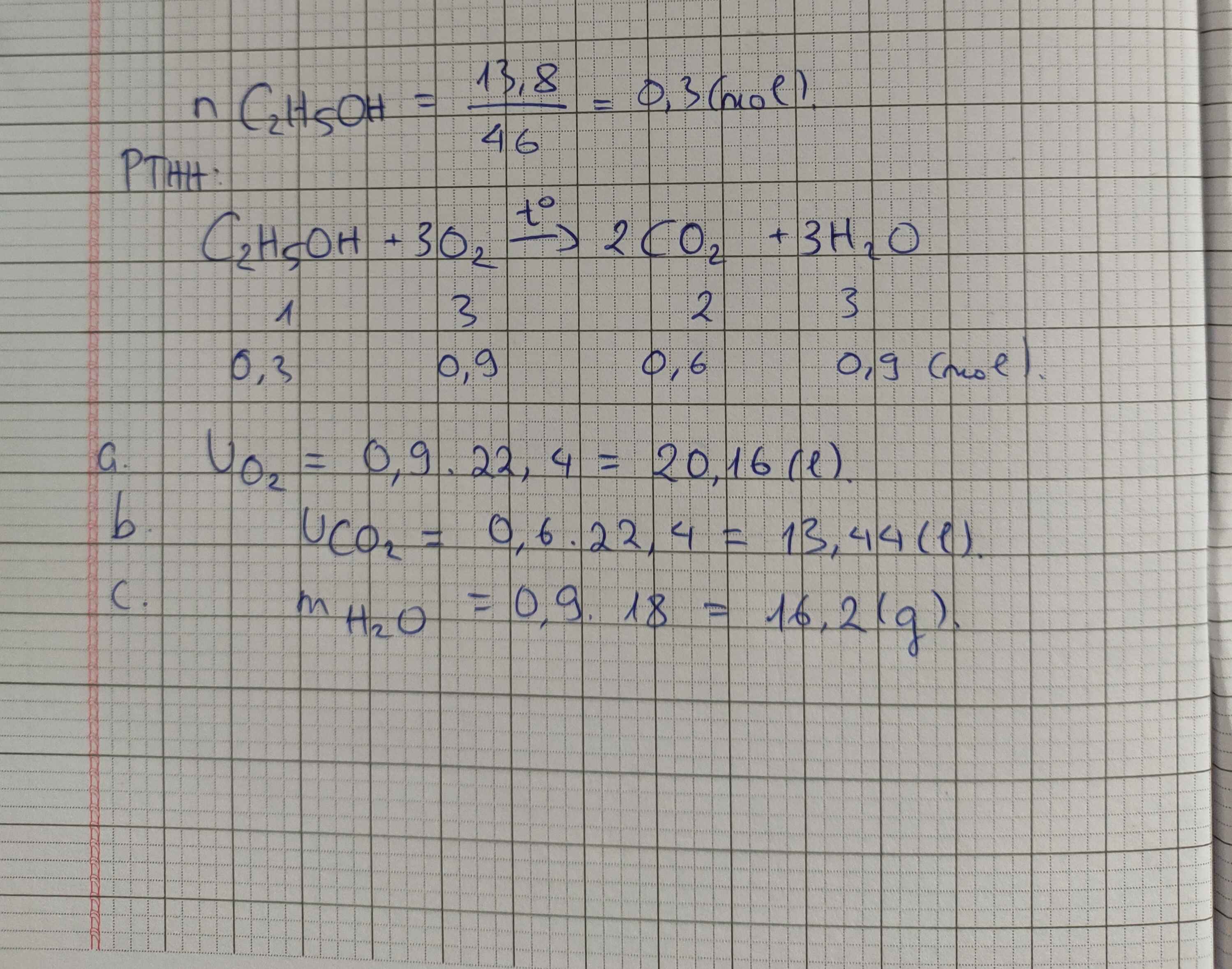
\(a,n_{C_2H_4}=\dfrac{30,9875}{24,79}=1,25\left(mol\right)\)
PTHH: C2H4 + 3O2 --to--> 2CO2 + 2H2O
1,25--->3,75
b, \(V_{O_2}=3,75.24,79=92,9625\left(l\right)\)
c, PTHH: C2H4 + H2O --axit--> C2H5OH
1,25--------------------->1,25
\(\Rightarrow m_{C_2H_5OH}=1,25.80\%.46=46\left(g\right)\)