Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Fe}=\dfrac{m_{Fe}}{M_{Fe}}=\dfrac{16,8}{56}=0,3mol\)
\(n_{O_2}=\dfrac{V_{O_2}}{22,4}=\dfrac{8,96}{22,4}=0,4mol\)
\(3Fe+2O_2\rightarrow\left(t^o\right)Fe_3O_4\)
0,3 < 0,4 ( mol )
0,3 0,2 0,1 ( mol )
Chất dư là \(O_2\)
\(n_{O_2\left(du\right)}=0,4-0,2=0,2mol\)
\(m_{Fe_3O_4}=n_{Fe_3O_4}.M_{Fe_3O_4}=0,1.232=23,2g\)

\(n_{Fe}=\dfrac{12.6}{56}=0.225\left(mol\right)\)
\(n_{O_2}=\dfrac{4.2}{22.4}=0.1875\left(mol\right)\)
\(3Fe+2O_2\underrightarrow{^{^{t^0}}}Fe_3O_4\)
\(3.........2\)
\(0.225......0.1875\)
Lập tỉ lệ : \(\dfrac{0.225}{3}< \dfrac{0.1875}{2}\Rightarrow O_2dư\)
\(m_{O_2\left(dư\right)}=\left(0.1875-0.225\cdot\dfrac{2}{3}\right)\cdot32=1.2\left(g\right)\)
\(m_{Fe_3O_4}=\dfrac{0.225}{3}\cdot232=17.4\left(g\right)\)

nFe = 16.8/56 = 0.3 (mol)
nO2 = 6.72/22.4 = 0.3 (mol)
2Fe + 3O2 -to-> Fe3O4
0.2___0.3________0.1
mFe dư = ( 0.3 - 0.2 ) * 56 = 5.6 (g)
mFe3O4 = 0.1*232 = 23.2 (g)
a)
3Fe+2O2→Fe3O4
b)
nFe=16,8/56=0,3mol
nO2=6,72/22,4=0,3mol
Ta có: 0,3/3<0,3/2=> O2 dư tính theo Fe
nFe3O4=0,3/3=0,1
mFe3O4=0,1.232=23,2g

\(n_P=\dfrac{6,2}{31}=0,2\left(mol\right)\\ n_{O_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\\ PTHH:4P+5O_2\underrightarrow{t^o}2P_2O_5\\ LTL:\dfrac{0,2}{4}< \dfrac{0,4}{5}\Rightarrow O_2dư\)
\(n_{O_2\left(pư\right)}=\dfrac{5}{4}n_P=\dfrac{5}{4}.0,2=0,25\left(mol\right)\\ n_{O_2\left(dư\right)}=0,4-0,25=0,15\left(mol\right)\)
\(n_{P_2O_5\left(lt\right)}=\dfrac{1}{2}n_P=\dfrac{1}{2}.0,2=0,1\left(mol\right)\\ m_{P_2O_5\left(lt\right)}=0,1.142=14,2\left(g\right)\\ m_{P_2O_5\left(tt\right)}=0,1.142.80\%=11,36\left(g\right)\)

\(a)3Fe+2O_2\rightarrow Fe_3O_4\)
\(3mol\) \(2mol\) \(1mol\)
\(0,3mol\) \(0,2mol\) \(0,1mol\)
\(b)n_{Fe}=\dfrac{m}{M}=\dfrac{16,8}{56}=0,3\left(mol\right)\)
\(n_{O_2}=\dfrac{V}{22,4}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
\(\text{Ta thấy }O_2\text{ dư,}Fe\text{ phản ứng hết}\)
\(c)m_{Fe_3O_4}=n.M=0,1.232=23,2\left(g\right)\)

\(n_P=\dfrac{3,1}{31}=0,1\left(mol\right)\)
\(n_{O_2}=\dfrac{4,48}{22,4}=0,2mol\)
4P + 5O2 \(\underrightarrow{t^o}\) 2P2O5
\(\dfrac{0,1}{4}< \dfrac{0,2}{5}\) => O2 dư, Photpho đủ
\(n_{O_2}=0,2-0,04=0,16\left(mol\right)\)
\(m_{P_2O_5}=\) 0,05 . 142 = 7,1 ( g )

\(n_{Zn}=\dfrac{13}{65}=0.2\left(mol\right)\)
\(n_{O_2}=\dfrac{8.96}{22.4}=0.4\left(mol\right)\)
\(2Zn+O_2\underrightarrow{^{^{t^0}}}2ZnO\)
LTL : \(\dfrac{0.2}{2}< \dfrac{0.4}{1}\Rightarrow O_2dư\)
\(m_{O_2\left(dư\right)}=\left(0.4-0.1\right)\cdot32=9.6\left(g\right)\)
\(m_{ZnO}=0.2\cdot81=16.2\left(g\right)\)

Sửa đề: 6,75 (l) → 6,72 (l)
a, \(n_{Al}=\dfrac{13,5}{27}=0,5\left(mol\right)\)
\(n_{O_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
PT: \(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
Xét tỉ lệ: \(\dfrac{0,5}{4}>\dfrac{0,3}{1}\), ta được Al dư.
Theo PT: \(n_{Al\left(pư\right)}=\dfrac{4}{3}n_{O_2}=0,4\left(mol\right)\Rightarrow n_{Al\left(dư\right)}=0,5-0,4=0,1\left(mol\right)\)
\(\Rightarrow m_{Al\left(dư\right)}=0,1.27=2,7\left(g\right)\)
b, Theo PT: \(n_{Al_2O_3}=\dfrac{2}{3}n_{O_2}=0,2\left(mol\right)\Rightarrow m_{Al_2O_3}=0,2.102=20,4\left(g\right)\)
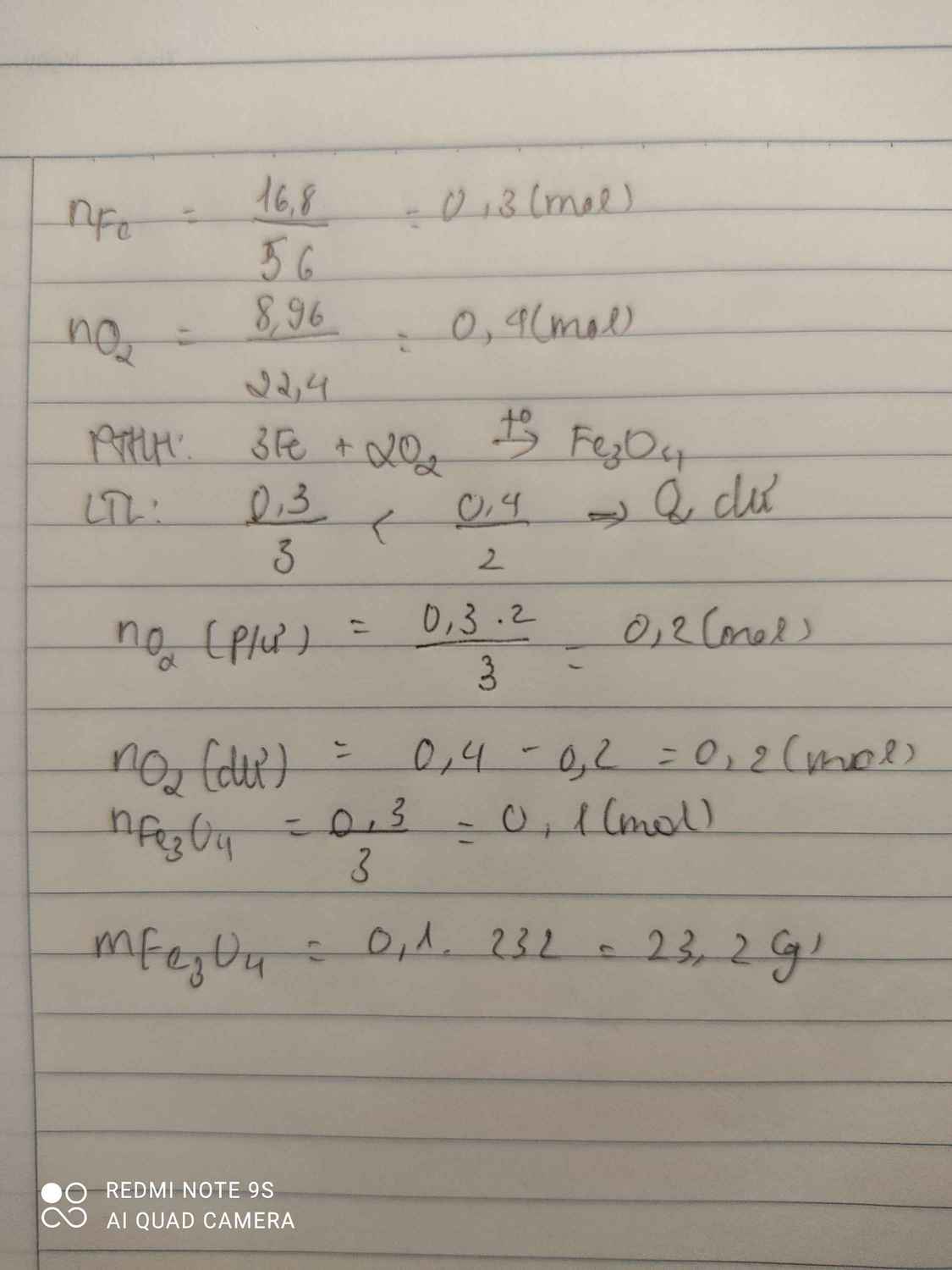
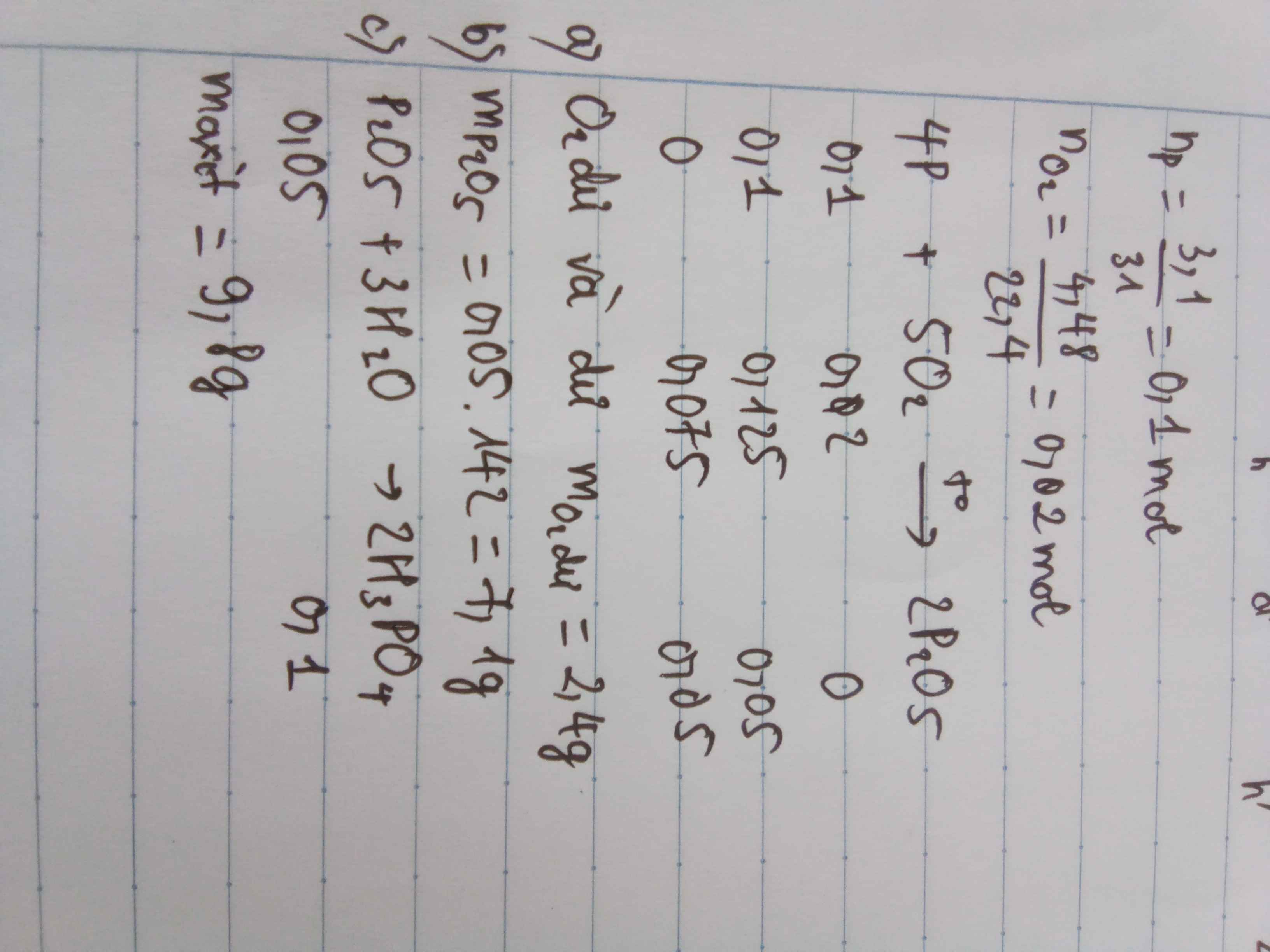
:V