Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(a,Đặt:n_{CH_4}=a\left(mol\right);n_{C_4H_{10}}=b\left(mol\right)\left(a,b>0\right)\\ PTHH:CH_4+2O_2\rightarrow\left(t^o\right)CO_2+2H_2O\\ 2C_4H_{10}+13O_2\rightarrow\left(t^o\right)8CO_2+10H_2O\\ \Rightarrow\left\{{}\begin{matrix}16a+58b=7,4\\22,4a+22,4.4b=22\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,1\\b=0,1\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}m_{CH_4}=0,1.16=1,6\left(g\right)\\m_{C_4H_{10}}=0,1.58=5,8\left(g\right)\end{matrix}\right.\\ b,n_{O_2}=2a+\dfrac{13}{2}b=2.0,1+6,5.0,1=0,85\left(mol\right)\\ \Rightarrow V_{O_2\left(đktc\right)}=0,85.22,4=19,04\left(l\right)\)

\(n_{O_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
PTHH: 2KMnO4 --to--> K2MnO4 + MnO2 + O2
1<------------------------------------0,5
=> \(m_{KMnO_4\left(pthh\right)}=1.158=158\left(g\right)\)
=> \(m_{KMnO_4\left(tt\right)}=\dfrac{158.100}{80}=197,5\left(g\right)\)

$n_{FeS_2} = \dfrac{36}{120} = 0,3(mol)$
$n_{O_2} = \dfrac{13,44}{22,4} = 0,6(mol)$
$4FeS_2 + 11O_2 \xrightarrow{t^o} 2Fe_2O_3 + 8SO_2$
Vì \(\dfrac{n_{FeS_2}}{4} = 0,075 > \dfrac{n_{O_2}}{11} = 0,0545\) nên $FeS_2$ dư
Gọi hiệu suất là a
\(n_{O_2\ pư} = 0,6a(mol)\\ n_{FeS_2} = \dfrac{4}{11}n_{O_2\ pư} = \dfrac{12a}{55}(mol)\\ n_{Fe_2O_3} = \dfrac{2}{11}n_{O_2\ pư} = \dfrac{6a}{55}(mol)\)
Suy ra :
120.(0,3 - 12a/55 )+ 160.6a/55 = 28
Suy ra a = 0,9167 = 91,67%
Sau phản ứng , khí gồm :
O2 dư : 0,6 - 0,6a = 0,05(mol)
SO2 : 0,4(mol)
Suy ra :
%V O2 = 0,05/(0,05 + 0,4) .100% = 11,11%
%V SO2 = 100% - 11,11% = 88,89%

a) \(n_{CH_4}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\)
\(n_{CO_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
PTHH: CH4 + 2O2 --to--> CO2 + 2H2O
0,05-->0,1------->0,05
2C2H2 + 5O2 --to--> 4CO2 + 2H2O
0,125<--0,3125<----0,25
=> \(\left\{{}\begin{matrix}\%V_{CH_4}=\dfrac{0,05}{0,05+0,125}.100\%=28,57\%\\\%V_{C_2H_2}=\dfrac{0,125}{0,05+0,125}.100\%=71,43\%\end{matrix}\right.\)
\(\left\{{}\begin{matrix}\%m_{CH_4}=\dfrac{0,05.16}{0,05.16+0,125.26}.100\%=19,753\%\\\%m_{C_2H_2}=\dfrac{0,125.26}{0,05.16+0,125.26}.100\%=80,247\%\end{matrix}\right.\)
b) \(n_{O_2}=0,1+0,3125=0,4125\left(mol\right)\)
=> \(V_{O_2}=0,4125.22,4=9,24\left(l\right)\)
=> Vkk = 9,24.5 = 46,2 (l)

a, \(CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\)
\(C_2H_4+3O_2\underrightarrow{t^o}2CO_2+2H_2O\)
\(2C_2H_2+5O_2\underrightarrow{t^o}4CO_2+2H_2O\)
b, Sửa đề: 17,9 (l) → 17,92 (l)
Ta có: \(n_{CO_2}=\dfrac{17,92}{22,4}=0,8\left(mol\right)=n_C\)
\(n_{H_2O}=\dfrac{18}{18}=1\left(mol\right)\Rightarrow n_H=1.2=2\left(mol\right)\)
⇒ mA = mC + mH = 0,8.12 + 2.1 = 11,6 (g)
Theo ĐLBT KL, có: mA + mO2 = mCO2 + mH2O
⇒ mO2 = 0,8.44 + 18 - 11,6 = 41,6 (g)
\(\Rightarrow n_{O_2}=\dfrac{41,6}{32}=1,3\left(mol\right)\Rightarrow V_{O_2}=1,3.22,4=29,12\left(l\right)\)

Câu 1:
\(m_{CaO(\text {phản ứng})}=\dfrac{50,4}{90\%}=56(g)\\ \Rightarrow n_{CaO}=\dfrac{56}{56}=1(mol)\\ PTHH:CaCO_3\xrightarrow{t^o}CaO+CO_2\\ \Rightarrow n_{CaCO_3}=1(mol)\\ \Rightarrow m_{CaCO_3}=100.1=100(g)\\ \Rightarrow m_{\text {đá vôi}}=\dfrac{100}{90\%}\approx 111,11(g)\)
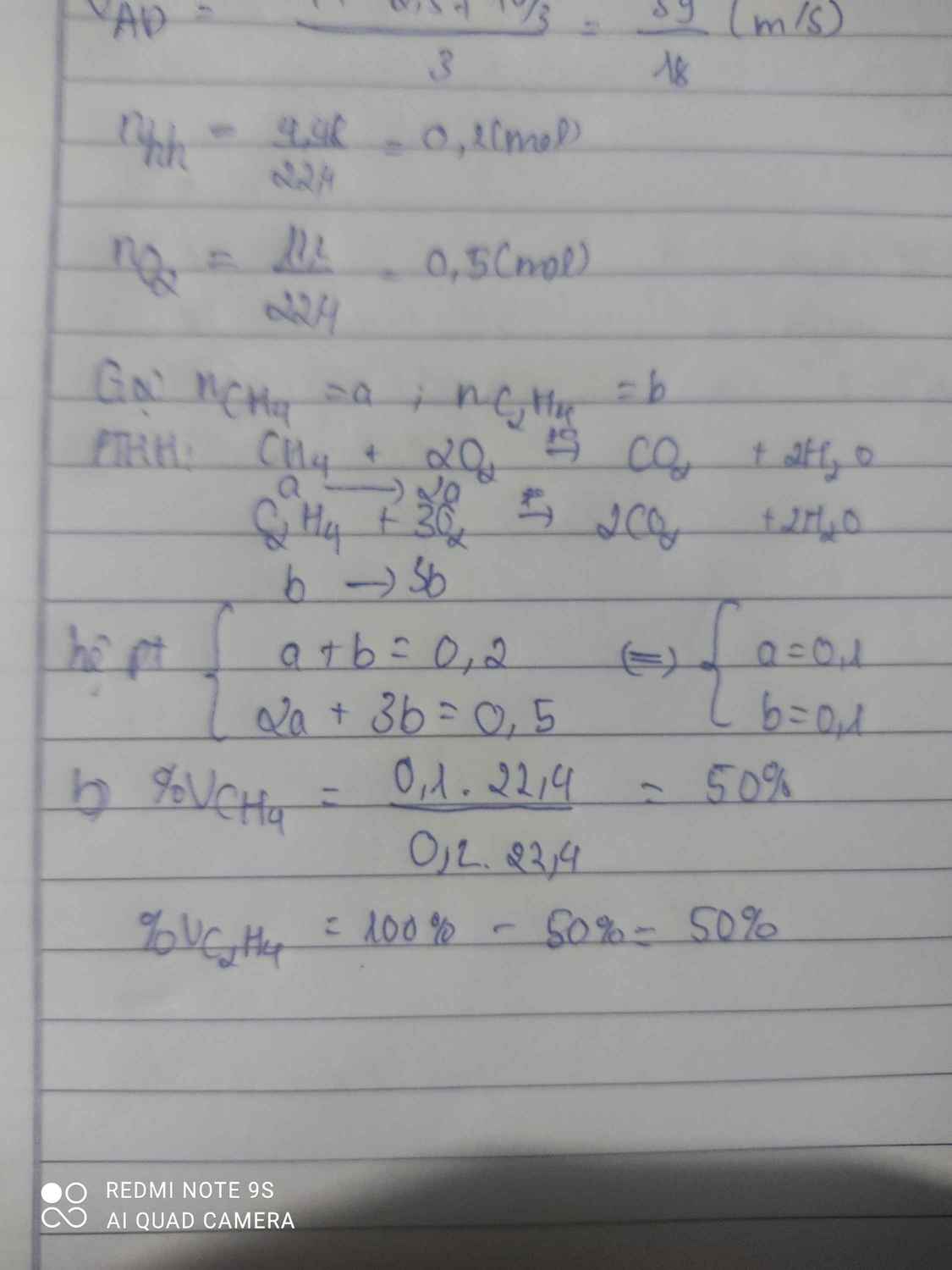
a)
\(\left\{{}\begin{matrix}n_{C_2H_2}+n_{CH_4}=\dfrac{13,44}{22,4}=0,6\left(mol\right)\\\dfrac{n_{C_2H_2}}{n_{CH_4}}=2\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}n_{C_2H_2}=0,4\left(mol\right)\\n_{CH_4}=0,2\left(mol\right)\end{matrix}\right.\)
PTHH: 2C2H2 + 5O2 --to--> 4CO2 + 2H2O
0,4----->1------------->0,8
CH4 + 2O2 --to--> CO2 + 2H2O
0,2-->0,4---------->0,2
=> VO2 = (1+0,4).22,4 = 31,36(l)
=> VCO2 = (0,8 + 0,2).22,4 = 22,4 (l)
b)
PTHH: 2KMnO4 --to--> K2MnO4 + MnO2 + O2
2,8<---------------------------------1,4
=> \(m_{KMnO_4\left(PTHH\right)}=2,8.158=442,4\left(g\right)\)
=> mKMnO4 (thực tế) = 442,4 : 80% = 553(g)
thanks nha :33