
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


a) \(C_xH_yCOOH\) + \(\left(x+\frac{y}{4}+\frac{1}{4}\right)O_2\rightarrow\left(x+1\right)CO_2+\left(\frac{y}{2}+\frac{1}{2}\right)H_2O\)
b) \(C_xH_y\left(COOH\right)_2+\left(x+\frac{y}{4}+\frac{1}{2}\right)O_2\rightarrow\left(x+1\right)CO_2+\left(\frac{y}{2}+1\right)H_2O\)

4CxHy(COOH)2 + (2x+y+2)O2 -------> (4x+8)CO2 + (2y+4)H2O

1.\(Fe_xO_y+2yHCl-->xFeCl_{\dfrac{2y}{x}}+yH_2O\)
2.\(2C_xH_y+\left(\dfrac{4x+y}{2}\right)O_2-->2xCO_2+yH_2O\)
3.\(C_nH_{2n}+\dfrac{3n}{2}O_2-->nCO_2+nH_2O\)
4.\(C_nH_{2n+2}+\left(\dfrac{3n+1}{2}\right)O_2-->nCO_2+\left(n+1\right)H_2O\)
5. \(2C_xH_yO_z+\left(\dfrac{4x+y-2z}{2}\right)O_2-->2xCO_2+yH_2O\)
6. (pthh này giống pt 3)

\(\begin{array}{l} 1,\ xFe_2O_3+(3x-2y)CO\xrightarrow{t^o} 2Fe_xO_y+(3x-2y)CO_2\uparrow\\ 2,\ C_xH_y+\bigg(x+\dfrac{y}{4}\bigg)O_2\xrightarrow{t^o} xCO_2\uparrow+\dfrac{y}{2}H_2O\end{array}\)

a) $4P + 5O_2 \xrightarrow{t^o} 2P_2O_5$
b) $Mg + Cl_2 \xrightarrow{t^o} MgCl_2$
c) $2Na + 2H_2O \to 2NaOH + H_2$
d) $C + O_2 \xrightarrow{t^o} CO_2$
e) $C_xH_y + (x + \dfrac{y}{4})O_2 \xrightarrow{t^o} xCO_2 + \dfrac{y}{2}H_2O$
f) $2Al + Fe_2O_3 \xrightarrow{t^o} Al_2O_3 + 2Fe$
g) $2Al + 3H_2SO_4 \to Al_2(SO_4)_3 + 3H_2$
i) $Fe_xO_y + yCO \xrightarrow{t^o} xFe + yCO_2$
k) $Fe_2O_3 + 6HCl \to 2FeCl_3 +3 H_2O$
l) $3Fe + 2O_2 \xrightarrow{t^o} Fe_3O_4$
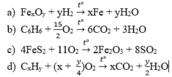
4CxHy(COOH)2 + (2x+y+2)O2 → (2y+4)H2O + (4x+8)CO2