Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a,\(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
PTHH: Mg + H2SO4 → MgSO4 + H2
Mol: 0,1 0,1 0,1
PTHH: MgO + H2SO4 → MgSO4 + H2O
Mol: 0,2 0,2
\(m_{Mg}=0,1.24=2,4\left(g\right)\)
\(m_{MgO}=10,4-2,4=8\left(g\right)\Rightarrow n_{MgO}=\dfrac{8}{40}=0,2\left(mol\right)\)
b,\(n_{MgSO_4}=0,1+0,2=0,3\left(mol\right)\)
PTHH: MgSO4 + 2NaOH → Mg(OH)2 ↓ + Na2SO4
Mol: 0,3 0,3
PTHH: Mg(OH)2 ---to→ MgO + H2O
Mol: 0,3 0,3
\(\Rightarrow m_{MgO}=0,3.40=12\left(g\right)\)

a) \(n_{NACl}=\frac{5,85}{58,5}=0,1\left(mol\right)\)
\(n_{AgNO_3}=\frac{34}{170}=0,2\left(mol\right)\)
\(NaCl+AgNO_3\rightarrow AgCl\downarrow+NaNO_3\)
0,1 \(\rightarrow\) 0,1 \(\rightarrow\) 0,1 \(\rightarrow\) 0,1 (mol)
\(m_{AgCl}=143,5.0,1=14,35g\)
b) \(V_{dd}=300+200=500\left(ml\right)\)
\(C_M\left(NaNO_3\right)=C_M\left(AgNO_3\right)=\frac{0,1}{0,5}=0,2\left(M\right)\)
a)nNaCl=0,1 mol , nAgNO3=0,2 mol
NaCl+AgNO3---->AgCl+NaNO3
theo pt và theo bài ra: NaCl hết, AgNO3 dư 0,1 mol
=> nAgCl=nNaCl=0,1=>mAgCl=14,35 gam.
b) thể tích sau phản ứng=200+300=500 ml= 0,5 lít
Nồng độ CMAgNO3=CMNaNO3=0,1/0,5=0,2.

1)
- TN1:
\(n_{AgCl}=\dfrac{35,875}{143,5}=0,25\left(mol\right)\)
PTHH: AgNO3 + HCl --> AgCl + HNO3
0,25<--0,25
TN2:
nNaOH = 0,5.0,3 = 0,15 (mol)
PTHH: NaOH + HCl --> NaCl + H2O
0,15--->0,15
\(n_{HCl\left(dd.C\right)}=0,25+0,15\) = 0,4 (mol)
=> \(C_{M\left(dd.C\right)}=\dfrac{0,4}{2}=0,2M\)
2)
Có \(\left\{{}\begin{matrix}C_{M\left(A\right)}=\dfrac{0,25}{V}M\\C_{M\left(B\right)}=\dfrac{0,15}{V^,}M\end{matrix}\right.\)
nHCl(A) = \(\dfrac{0,025}{V}\left(mol\right)\)
PTHH: Fe + 2HCl --> FeCl2 + H2
\(\dfrac{0,025}{V}\)------>\(\dfrac{0,0125}{V}\)
nHCl(B) = \(\dfrac{0,015}{V^,}\) (mol)
PTHH: Fe + 2HCl --> FeCl2 + H2
\(\dfrac{0,015}{V^,}\)-------->\(\dfrac{0,0075}{V^,}\)
TH1: \(\dfrac{0,0125}{V}=\dfrac{0,0075}{V^,}+0,02\)
Mà V + V' = 2 (l)
=> \(\left[{}\begin{matrix}V=1,5;V^,=0,5\left(KTM\right)\\V=0,5;V^,=1,5\left(TM\right)\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}C_{M\left(A\right)}=\dfrac{0,25}{0,5}=0,5M\\C_{M\left(B\right)}=\dfrac{0,15}{1,5}=0,1M\end{matrix}\right.\)
TH2: \(\dfrac{0,0125}{V}+0,02=\dfrac{0,0075}{V^,}\)
=> \(\left[{}\begin{matrix}V=\dfrac{1+\sqrt{6}}{2};V^,=\dfrac{3-\sqrt{6}}{2}\\V=\dfrac{1-\sqrt{6}}{2}\left(L\right)\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}C_{M\left(A\right)}=\dfrac{0,25}{\dfrac{1+\sqrt{6}}{2}}=\dfrac{-1+\sqrt{6}}{10}M\\C_{M\left(B\right)}=\dfrac{0,15}{\dfrac{3-\sqrt{6}}{2}}=\dfrac{3+\sqrt{6}}{10}M\end{matrix}\right.\)

nNaCl = = 0,1 mol;
=
= 0,2 mol
a) Phương trình hóa học của phản ứng:
NaCl + AgNO3 → AgCl↓ + NaNO3
0,1 mol 0,1 mol 0,1 mol 0,1 mol
mAgCl = 143,5 x 0,1 = 14,35g
b) Vdd = 300 + 200 = 500 ml
= 0,2 - 0,1 = 0,1 mol
=
=
= 0,2 mol/l
\(n_{NaCl}=\frac{5,85}{58,5}=0,1\left(mol\right)\)
\(n_{AgNO_3}=\frac{34}{170}=0,2\left(mol\right)\)
\(NaCl+AgNO_3->AgCl+NaNO_3\) (1)
vì \(\frac{0,1}{1}< \frac{0,2}{1}\) => \(AgNO_3dư\)
theo (1) \(n_{AgCl}=n_{NaCl}=0,1\left(mol\right)\)
=> \(m_{AgCl}=143,5.0,1=14,35\left(g\right)\)
b, 300ml=0,3l , 200ml = 0,2 l
\(V_{dd}=0,3+0,2=0,5\left(l\right)\)
theo (1) \(n_{AgNO_3\left(pư\right)}=n_{NaCl}=0,1\left(mol\right)\)
=> \(n_{AgNO_3\left(dư\right)}=0,2-0,1=0,1\left(mol\right)\)
\(C_{M\left(NaNO_3\right)}=\frac{0,1}{0,5}=0,2M\)

Chúc bạn học tốt !!!
\(m_{hh}=74.5a+58.5b=26.6\left(g\right)\left(1\right)\)
\(n_{AgCl}=\dfrac{57.4}{143.5}=0.4\left(mol\right)\)
\(KCl+AgNO_3\rightarrow KNO_3+AgCl\)
\(NaCl+AgNO_3\rightarrow NaNO_3+AgCl\)
\(n_{AgCl}=a+b=0.4\left(mol\right)\left(2\right)\)
\(\left(1\right),\left(2\right):\)
\(a=b=0.2\)
\(m_{dd\left(saupư\right)}=26.6+500-57.4=469.2\left(g\right)\)
\(C\%_{KNO_3}=\dfrac{0.2\cdot101}{469.2}\cdot100\%=4.31\%\)
\(C\%_{NaNO_3}=\dfrac{0.2\cdot85}{469.2}\cdot100\%=3.62\%\)
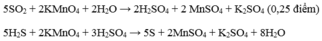

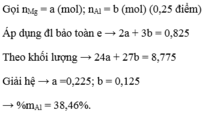
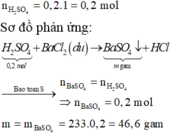
\(n_{BaSO_4}=0,2\left(mol\right)\\ BTNT.S\Rightarrow n_{H_2SO_4}=n_{BaSO_4}=0,2\left(mol\right)\)
\(OH^-+H^+\rightarrow H_2O\)
0,8_____0,8
\(\Rightarrow n_{H^+}=2n_{H_2SO_4}+n_{HCl}\Rightarrow n_{HCl}=0,4\left(mol\right)\)
\(H_2SO_4+BaCl_2\rightarrow BaSO_4+2HCl\left(1\right)\)
\(HCl+NaOH\rightarrow NaCl+H_2O\left(2\right)\)
Ta có:
\(n_{H2SO4}=n_{BaSO4}=\frac{46,6}{233}=0,2\left(mol\right)\)
\(\Rightarrow n_{HCl\left(1\right)}=0,4\left(mol\right)\)
\(n_{HCl\left(2\right)}=0,5.1,6=0,8\left(mol\right)\)
\(\Rightarrow n_{HCl}=0,8-0,4=0,4\left(mol\right)\)
\(C\%_{HCl}=\frac{0,4.36,5}{200}.100\%=7,3\%\)
\(C\%_{H2SO4}=\frac{0,2.98}{200}.100\%=9,8\%\)