Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(\left(a\right)2Al+3H_2O\rightarrow Al_2\left(SO_4\right)_3+3H_2\\ \left(b\right)n_{H_2}=\dfrac{13,44}{22,4}=0,6mol\\ n_{Al}=\dfrac{0,6.2}{3}=0,4mol\\ m_{Al}=0,4.27=10,8g\\ \left(c\right)n_{O_2}=\dfrac{4,8}{32}=0,15mol\\ 4Al+3O_2\underrightarrow{t^0}2Al_2O_3\\ \Rightarrow\dfrac{0,4}{4}>\dfrac{0,15}{3}\Rightarrow Al.dư\\ n_{Al_2O_3}=\dfrac{0,15.2}{3}=0,1mol\\ m_{oxit}=m_{Al_2O_3}=0,1.102=10,2g\)
a: \(2Al+3H_2SO_4\rightarrow1Al_2\left(SO_4\right)_3+3H_2\uparrow\)
0,4 0,6 0,2 0,6
b: \(n_{H_2}=\dfrac{13.44}{22.4}=0.6\left(mol\right)\)
=>\(n_{Al}=0.4\left(mol\right)\)
\(m_{Al}=0.4\cdot27=10.8\left(g\right)\)
c: \(4Al+3O_2\rightarrow2Al_2O_3\)
0,4 0,2
\(m_{Al_2O_3}=0.2\left(27\cdot2+16\cdot3\right)=0.2\cdot102=20.4\left(g\right)\)

Đặt số mol Fe3O4 là x (mol)
Fe3O4 + 8HCl → 2FeCl3 + FeCl2 + 4H2O
x..............8x..........2x............x
Cu + 2FeCl3 ⟶ 2FeCl2 + CuCl2
x.........2x................2x.............x
Kim loại không tan là Cu
Dung dịch Y gồm FeCl2, CuCl2 và HCl dư
=> \(n_{FeCl_2}=x+2x=3x\left(mol\right);n_{CuCl_2}=x\left(mol\right)\)
\(n_{OH^-}=0,5.1+0,5.1=1\left(mol\right)\)
\(H^+_{\left(dư\right)}+OH^-\rightarrow H_2O\)
\(Fe^{2+}+2OH^-\rightarrow Fe\left(OH\right)_2\)
3x..........6x...............3x
\(Cu^{2+}+2OH^-\rightarrow Cu\left(OH\right)_2\)
x.............2x.................x
Kết tủa là Cu(OH)2 và Fe(OH)2
Ta có : \(3x.90+x.98=36,8\)
=> x=0,1 (mol)
=> \(m_{Cu}=x.64+1,6=8\left(g\right)\)
=> \(m=0,1.232+8=31,2\left(g\right)\)
Mặt khác : \(n_{HCl\left(dư\right)}=1-\left(6x+2x\right)=0,2\left(mol\right)\)
=> \(n_{HCl\left(bđ\right)}=8x+0,2=1\left(mol\right)\)

\(PTHH:Fe+CuSO_4\)→\(FeSO_4+Cu\)
\(+n_{Fe}=\dfrac{16,8}{56}=0,3\left(mol\right)\)
Theo PTHH ta có:
\(+n_{Cu}=n_{Fe}=0,3\left(mol\right)\)
\(+m_{Cu}=0,3.64=19,2\left(gam\right)\)
\(+n_{CuSO_4}=n_{Fe}=0,3\left(mol\right)\)
\(+V_{CuSO_4}=0,3.0,5=0,15\left(lit\right)\)
d)
PTHH: \(2NaOH+CuSO_4\) →\(Cu\left(OH\right)_2\downarrow+Na_2SO_4\)
Cu(OH)2 làm quỳ tím chuyển màu xanh vì là bazo.

\(a,2NaOH+MgSO_4\rightarrow Mg\left(OH\right)_2+Na_2SO_4\\ n_{NaOH}=0,5.1=0,5\left(mol\right)\\ b,n_{Mg\left(OH\right)_2}=\dfrac{0,5}{2}=0,25\left(mol\right)=n_{Na_2SO_4}\\ m_{kt}=m_{Mg\left(OH\right)_2}=58.0,25=14,5\left(g\right)\\ c,V_{ddX}=V_{ddNaOH}+V_{ddMgSO_4}=0,5+0,5=1\left(l\right)\\ C_{MddNa_2SO_4}=\dfrac{0,25}{1}=0,25\left(M\right)\)

a) Zn+ CuSO4 -> ZnSO4 + Cu
Ta có: nZn=0,02(mol)
b) nCu=nZn=nZnSO4=0,02(mol)
=>mCu=0,02.64=1,28(g)
c) mZnSO4=161.0,02=3,22(g)
mddZnSO4=mZn+mddCuSO4-mCu= 1,3+20-1,28=20,02(g)
=>C%ddZnSO4= (3,22/20,02).100=16,084%

a, \(2NaOH+CuSO_4\rightarrow Na_2SO_4+Cu\left(OH\right)_2\)
\(Cu\left(OH\right)_2\underrightarrow{t^o}CuO+H_2O\)
b, \(m_{CuSO_4}=250.16\%=40\left(g\right)\Rightarrow n_{CuSO_4}=\dfrac{40}{160}=0,25\left(mol\right)\)
Theo PT: \(n_{CuO}=n_{Cu\left(OH\right)_2}=n_{CuSO_4}=0,25\left(mol\right)\)
\(\Rightarrow a=m_{CuO}=0,25.80=20\left(g\right)\)
c, Ta có: m dd sau pư = m dd NaOH + m dd CuSO4 - mCu(OH)2 = 200 + 250 - 0,25.98 = 425,5 (g)

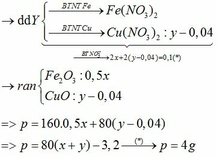
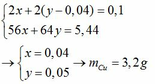
Ta có: \(n_{CuSO_4}=0,3\left(mol\right)\)
a, PT: \(2Al+3CuSO_4\rightarrow Al_2\left(SO_4\right)_3+3Cu\)
______0,2____0,3_________________0,3 (mol)
b, \(m_{Al}=0,2.27=5,4\left(g\right)\)
c, \(m_{Cu}=0,3.64=19,2\left(g\right)\)
Bạn tham khảo nhé!