Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

n C2H5OH =a (mol) ; n CH3COOH = b(mol)
=> 46a + 60b = 27,2(1)
$2C_2H_5ONa + 2Na \to 2C_2H_5ONa + H_2$
$2CH_3COOH + 2Na \to 2CH_3COONa + H_2$
Theo PTHH :
n H2 = 0,5a + 0,5b = 5,6/22,4 = 0,25(2)
Từ (1)(2) suy ra a = 0,2 ; b = 0,3
Suy ra:
m C2H5OH = 0,2.46 = 9,2(gam)
m CH3COOH = 0,3.60 = 18(gam)

nH2 = 85,12 : 22,4 = 3,8 (mol) ; nH2O = VH2O.D = 108 (g) => nH2O = 108/18 = 6 (mol)
PTHH:
2Na + 2C2H5OH → 2C2H5ONa + H2↑
x → 0,5x (mol)
2Na + 2H2O → 2NaOH + H2↑
6 → 3 (mol)
Ta có: nH2 = 0,5x + 3 = 3,8
=> x = 1,6 (mol) = nC2H5OH
mC2H5OH = 1,6.46 = 73,6 (g)
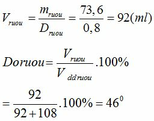

\(n_{H_2}=\dfrac{11,2}{22,4}=0,5mol\)
\(2C_2H_5OH+2Na\rightarrow2C_2H_5ONa+H_2\)
1 0,5 ( mol )
\(C_2H_5OH+O_2\rightarrow\left(men.giấm\right)CH_3COOH+H_2O\)
1 1 ( mol )
\(m_{CH_3COOH}=1.60.80\%=48g\)

\(n_{C_2H_5OH}=\dfrac{14}{46}=\dfrac{7}{23}\left(mol\right)\)
\(C_2H_5OH+Na\rightarrow C_2H_5ONa+\dfrac{1}{2}H_2\)
\(\dfrac{7}{23}...................\dfrac{7}{23}......\dfrac{7}{46}\)
\(m_{C_2H_5ONa}=\dfrac{7}{23}\cdot68=20.7\left(g\right)\)
\(V_{H_2}=\dfrac{7}{46}\cdot22.4=3.4\left(l\right)\)
\(a) 2C_2H_5OH + 2Na \to 2C_2H_5ONa + H_2\\ n_{C_2H_5ONa} = n_{C_2H_5OH} = \dfrac{14}{46} = \dfrac{7}{23}(mol)\\ m_{C_2H_5ONa} = \dfrac{7}{23}.68 = 20,7(gam)\\ n_{H_2} = \dfrac{1}{2}n_{C_2H_5OH} = \dfrac{7}{46}(mol)\\ m_{H_2} = \dfrac{7}{46}.2 = \dfrac{7}{23}(gam)\\ b) V_{H_2} = \dfrac{7}{46}.22,4 = 3,41(lít)\)

\(nC_2H_5OH=\dfrac{2,9}{46}=0,06\left(mol\right)\)
\(C_2H_5OH+Na\rightarrow C_2H_5ONa+\dfrac{1}{2}H_2\)
0,06 0,06 0,06 0,03 (mol)
VH2 = 0,03.22,4= 0,672 (l)
V = m /D
=> V rượu etylic = 2,9 / 0,8 = 3,625 (ml)

\(n_{C_2H_4}=\dfrac{1,12}{22,4}=0,05mol\)
\(C_2H_4+H_2O\rightarrow\left(t^o,H_2SO_4\right)C_2H_5OH\)
0,05 0,05 ( mol )
\(C_2H_5OH+CH_3COOH\rightarrow\left(t^o,H_2SO_4\right)CH_3COOC_2H_5+H_2O\)
0,05 0,05 ( mol )
\(m_{CH_3COOC_2H_5}=0,05.88=4,4g\)

\(n_{H_2}=\dfrac{1,12}{22,4}=0,05mol\)
\(2C_2H_5OH+2Na\rightarrow2C_2H_5ONa+H_2\)
0,1 0,05 ( mol )
\(m_{C_2H_5OH}=0,1.46=4,6g\)
C2H5OH+Na->C2H5ONa+\(\dfrac{1}{2}\)H2
0,1---------------------------------0,05
=>n H2=0,05 mol
=>m C2H5OH=0,1.46=4,6g