Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{H_2}=\dfrac{V}{22,4}=\dfrac{4,958}{22,4}=\approx0,2\left(mol\right)\)
\(n_{O_2}=\dfrac{V}{22,4}=\dfrac{1,2395}{22,4}\approx0,05\left(mol\right)\)
\(PTHH:2H_2+O_2\rightarrow2H_2O\)
Ta có: \(\dfrac{n_{H_2}}{2}=\dfrac{0,2}{2}=0,1>\dfrac{n_{O_2}}{1}=\dfrac{0,05}{1}=0,05\)
→ Sau pư O2 hết, H2 dư
→ Theo \(n_{O_2}\)
Theo PTHH \(n_{H_2O}=2n_{O_2}=2.0,05=0,1\left(mol\right)\)
\(V_{H_2O\left(đktc\right)}=n.22,4=0,1.22,4=2,24\left(l\right)\)
Vậy ...

Câu 8:
Ta có: \(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
\(n_{O_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
PT: \(2H_2+O_2\underrightarrow{t^o}2H_2O\)
Xét tỉ lệ: \(\dfrac{0,1}{2}< \dfrac{0,2}{1}\), ta được O2 dư.
Theo PT: \(\left\{{}\begin{matrix}n_{O_2\left(pư\right)}=\dfrac{1}{2}n_{H_2}=0,05\left(mol\right)\\n_{H_2O}=n_{H_2}=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow n_{O_2\left(dư\right)}=0,15\left(mol\right)\)
\(\Rightarrow V_{O_2\left(dư\right)}=0,15.22,4=3,36\left(l\right)\)
\(m_{H_2O}=0,1.18=1,8\left(g\right)\)
Bạn tham khảo nhé!
Câu 9:
a, PT: \(2R+O_2\underrightarrow{t^o}2RO\)
Theo ĐLBT KL, có: mR + mO2 = mRO
⇒ mO2 = 4,8 (g)
\(\Rightarrow n_{O_2}=\dfrac{4,8}{32}=0,15\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,15.22,4=3,36\left(l\right)\)
b, Theo PT: \(n_R=2n_{O_2}=0,3\left(mol\right)\)
\(\Rightarrow M_R=\dfrac{19,2}{0,3}=64\left(g/mol\right)\)
Vậy: M là đồng (Cu).
Câu 10:
Ta có: mBaCl2 = 200.15% = 30 (g)
a, m dd = 200 + 100 = 300 (g)
\(\Rightarrow C\%_{BaCl_2}=\dfrac{30}{300}.100\%=10\%\)
⇒ Nồng độ dung dịch giảm 5%
b, Ta có: \(C\%_{BaCl_2}=\dfrac{30}{150}.100\%=20\%\)
⇒ Nồng độ dung dịch tăng 5%.
Bạn tham khảo nhé!

\(a) Fe + 2HCl \to FeCl_2 + H_2\\ n_{H_2} = n_{Fe} = \dfrac{5,6}{56} = 0,1(mol)\\ \Rightarrow V_{H_2} = 0,1.22,4 = 2,24(lít)\\ b) n_{O_2} = \dfrac{6,72}{22,4} = 0,3(mol)\\ 2H_2 + O_2 \xrightarrow{t^o} 2H_2O\\ \dfrac{n_{H_2}}{2} = 0,05 < \dfrac{n_{O_2}}{1} = 0,3 \to O_2\ dư\\ n_{H_2O} = n_{H_2} = 0,1(mol) \Rightarrow m_{H_2O} = 0,1.18 = 1,8(gam)\)

a) \(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\)
PTHH: Zn + 2HCl --> ZnCl2 + H2
0,1-->0,2------>0,1-->0,1
=> VH2 = 0,1.22,4 = 2,24 (l)
mZnCl2 = 0,1.136 = 13,6 (g)
b) \(C\%_{dd.HCl}=\dfrac{0,2.36,5}{200}.100\%=3,65\%\)
c) \(n_{O_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
PTHH: 2H2 + O2 --to--> 2H2O
Xét tỉ lệ: \(\dfrac{0,1}{2}< \dfrac{0,15}{1}\) => H2 hết, O2 dư
PTHH: 2H2 + O2 --to--> 2H2O
0,1--------------->0,1
=> mH2O = 0,1.18 = 1,8 (g)

\(a) Zn + 2HCl \to ZnCl_2 + H_2\\ n_{H_2} = n_{Zn} = \dfrac{13}{65} = 0,2(mol)\\ V_{H_2} = 0,2.22,4 = 4,48(lít)\\ b)n_{O_2} =\dfrac{4,8}{32}=0,15(mol)\\ 2H_2 + O_2 \xrightarrow{t^o} 2H_2O\\ \dfrac{n_{H_2}}{2} = 0,1 < \dfrac{n_{O_2}}{1} = 0,15 \to O_2\ dư\\ n_{H_2O} = n_{H_2} = 0,2(mol)\\ \Rightarrow m_{H_2O} = 0,2.18= 3,6(gam)\)

a, \(2H_2+O_2\underrightarrow{t^o}2H_2O\)
b, \(n_{H_2}=\dfrac{2,8}{22,4}=0,125\left(mol\right)\)
Theo PT: \(n_{O_2}=\dfrac{1}{2}n_{H_2}=0,0625\left(mol\right)\Rightarrow V_{O_2}=0,0625.22,4=1,4\left(l\right)\)
\(n_{H_2O}=n_{H_2}=0,125\left(mol\right)\Rightarrow m_{H_2O}=0,125.18=2,25\left(g\right)\)
Số mol của 2,8 lít khí H2
nH2 = \(\dfrac{V}{22,4}\) = \(\dfrac{2,8}{22,4}\) = 0.125 mol
a. PTHH: 2H2 + O2 \(\rightarrow\) 2H2O
Tỉ lệ: 2 1 2
Mol: 0.125 \(\rightarrow\) 0.1 \(\rightarrow\) 0.125
b. Thể tích khí O2 ở đktc
VO2 = n . 22,4 = 0.1 . 22,4 = 2,24 (l)
Khối lượng H2O thu được
mH2O = n . M = 0.125 . 18 = 2,25g

\(n_{O_2}=\dfrac{1,28}{32}=0,04\left(mol\right);n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\\ PTHH:2H_2+O_2\rightarrow\left(t^o\right)2H_2O\\ Vì:\dfrac{0,1}{2}>\dfrac{0,04}{1}\Rightarrow H_2dư\\ n_{H_2O}=2.n_{O_2}=2.0,04=0,08\left(mol\right)\\ m_{H_2O}=0,08.18=1,44\left(g\right)\)
\(V_{H_2}=2,24\left(l\right)\Rightarrow n_{H_2}=\dfrac{V_{H_2}}{22,4}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
\(PTHH:2H_2+O_2\underrightarrow{đp}2H_2O\)
\(pt:\) \(2mol\) \(2mol\)
\(đb:\) \(0,1mol\) \(\rightarrow\) \(0,1mol\)
\(\Rightarrow n_{H_2O}=0,1mol\)
\(m_{H_2O}=n_{H_2O}.M_{H_2O}=0,1.\left(2.H+1.O\right)=0,1.\left(2.1+1.16\right)=1,8\left(g\right)\)
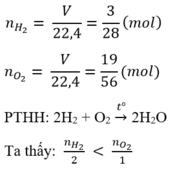
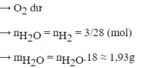
\(n_{H_2}=\dfrac{15,6}{22,4}=\dfrac{39}{56}\left(mol\right)\\ 2H_2+O_2\rightarrow\left(t^o\right)2H_2O\\ n_{H_2O}=n_{H_2}=\dfrac{39}{56}\left(mol\right)\\ m_{H_2O}=\dfrac{39}{56}.18\approx12,536\left(g\right)\)