Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a)
$Fe_2(SO_4)_3 + 6KOH \to 2Fe(OH)_3 + 3K_2SO_4$
b)
$n_{Fe_2(SO_4)_3} = 0,3.1 = 0,3(mol)$
$n_{KOH} = \dfrac{16,8}{56} =0,3(mol)$
Ta thấy :
$n_{KOH} : 3 < n_{Fe_2(SO_4)_3} : 1$ nên $Fe_2(SO_4)_3$ dư
$n_{Fe(OH)_3} = \dfrac{1}{3}n_{KOH} = 0,1(mol)$
$n_{Fe_2O_3} = \dfrac{1}{2}n_{Fe(OH)_3} = 0,05(mol)$
$m_{Fe_2O_3} = 0,05.160 = 8(gam)$

gọi x la so mol cua Fe
y la so mol cua FeO
\(n_{H_2SO_4}=0,3.1=0,3\left(mol\right)\)
Fe + H2SO4 \(\rightarrow\) FeSO4 + H2
de: x \(\rightarrow\) x \(\rightarrow\) x \(\rightarrow\) x
FeO + H2SO4 \(\rightarrow\) FeSO4 + H2
de: y \(\rightarrow\) y \(\rightarrow\) y \(\rightarrow\) y
Ta co: 56x + 72y = 18,4
x + y = 0,3
\(\Rightarrow\) x = 0,2 y = 0,1
a, \(m_{Fe}=56.0,2=11,2g\)
\(m_{FeO}=72.0,1=7,2g\)
\(\%m_{Fe}=\dfrac{11,2}{18,4}.100\%\approx60,87\%\)
\(\%m_{FeO}=100-60,87\approx39,13\%\)
b, \(m_{FeSO_4}=152.\left(0,1+0,2\right)=45,6g\)
\(m_{dd}=18,4+300.1,65-0,4=513g\)
\(C\%=\dfrac{45,6}{513}.100\%\approx8,89\%\)

Bảo toàn Cu: `n_{Cu}=n_{CuSO_4}={50.9,6\%}/{160}=0,03(mol)`
`->m_{Cu}=0,03.64=1,92<2,48`
`->Y` chứa `Fe` dư và `Cu.`
`->m_{Fe\ du}=2,48-1,92=0,56(g)`
`Mg+CuSO_4->MgSO_4+Cu`
`Fe+CuSO_4->FeSO_4+Cu`
Đặt `n_{Mg}=x(mol);n_{Fe\ pu}=y(mol)`
Theo PT: `n_{Cu}=x+y=0,03(1)`
`MgSO_4+2NaOH->Mg(OH)_2+Na_2SO_4`
`FeSO_4+2NaOH->Fe(OH)_2+Na_2SO_4`
`Mg(OH)_2` $\xrightarrow{t^o}$ `MgO+H_2O`
`4Fe(OH)_2+O_2` $\xrightarrow{t^o}$ `2Fe_2O_3+4H_2O`
Theo PT: `n_{MgO}=x(mol);n_{Fe_2O_3}=0,5y(mol)`
`->40x+160.0,5y=2(2)`
`(1)(2)->x=0,01;y=0,02`
`->m=0,01.24+0,02.56+0,56=1,92(g)`
`\%m_{Mg}={0,01.24}/{1,92}.100\%=12,5\%`
`\%m_{Fe}=100-12,5=87,5\%`
`m_{dd\ spu}=1,92+50-2,48=49,44(g)`
`Z` gồm `MgSO_4:0,01(mol);FeSO_4:0,02(mol)`
`->C\%_{MgSO_4}={0,01.120}/{49,44}.100\%\approx 2,43\%`
`C\%_{FeSO_4}={0,02.152}/{49,44}.100\%\approx 6,15\%`

\(a,PTHH:3NaOH+FeCl_3\rightarrow3NaCl+Fe\left(OH\right)_3\downarrow\\ 2Fe\left(OH\right)_3\rightarrow^{t^o}Fe_2O_3+3H_2O\uparrow\\ b,n_{FeCl_3}=1,5\cdot0,2=0,3\left(mol\right)\\ \Rightarrow n_{NaOH}=3n_{FeCl_3}=0,9\left(mol\right)\\ \Rightarrow V_{dd_{NaOH}}=\dfrac{0,9}{2}=0,45\left(l\right)\)
Theo đề: \(\left\{{}\begin{matrix}X:Fe\left(OH\right)_3\\A:NaCl\\Y:Fe_2O_3\end{matrix}\right.\)
Theo PT: \(n_{NaCl}=3n_{FeCl_3}=0,9\left(mol\right)\)
\(\Rightarrow C_{M_{NaCl}}=\dfrac{0,9}{0,45+0,2}\approx1,4M\)
\(c,\) Theo PT: \(n_{Fe\left(OH\right)_3}=n_{FeCl_3}=0,3\left(mol\right);n_{Fe_2O_3}=\dfrac{1}{2}n_{Fe\left(OH\right)_3}=0,15\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}m_X=m_{Fe\left(OH\right)_3}=0,3\cdot107=32,1\left(g\right)\\m_Y=m_{Fe_2O_3}=0,15\cdot160=24\left(g\right)\end{matrix}\right.\)

VFeCl2 = 100 ml = 0,1 (l)
=> nFeCl2 = 0,1 . 1 = 0,1 mol
FeCl2 + 2NaOH->2 NaCl + Fe(OH)2 \(\downarrow\)
0,1----------------------------->0,1 mol
Fe(OH)2 \(^{to}\rightarrow\) FeO + H2O
0,1---------->0,1mol
FeO + CO \(^{to}\rightarrow\) Fe + CO2 \(\uparrow\)
0,1--------------->0,1mol
=> mFe = 0,1 . 56 = 5,6 g
vậy khối lượng KL màu trắng bạc (Fe) là 5,6 g


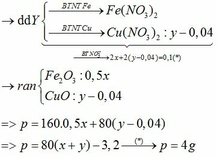
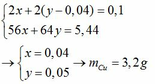
3KOH +FeCl3 --> Fe(OH)3 +3KCl(1)
2Fe(OH)3 -->Fe2O3 +3H2O(2)
Fe2O3 +3CO -->2Fe +3CO2(3)
nFeCl3=0,1.1=0,1(mol)
theo (2) : nFe2O3=1/2nFe(OH)3=0,05(mol)
theo (3) : nFe=2nFe2O3=0,1(mol)
=> mFe=0,1.56=5,6(g)