Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\\ PTHH:Zn+2HCl\rightarrow ZnCl_2+H_2\\ n_{H_2}=n_{ZnCl_2}=n_{Zn}=0,2\left(mol\right)\\ a,V_{H_2\left(Đktc\right)}=0,2.22,4=4,48\left(l\right)\\ b,m_{ZnCl_2}=136.0,2=27,2\left(g\right)\\ c,n_{HCl}=0,2.2=0,4\left(mol\right)\\ C\%_{ddHCl}=\dfrac{0,4.36,5}{200}.100\%=7,3\%\)

\(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\\
pthh:Zn+2HCl\rightarrow ZnCl_2+H_2\)
0,1 0,2 0,1 0,1
\(m_{HCl}=0,2.36,5=7,3\left(g\right)\\
V_{H_2}=0,1.22,4=2,24l\\
m_{\text{dd}}=6,5+200-\left(0,1.2\right)=206,3g\)
bài 2 :
\(n_{Mg}=\dfrac{4,8}{24}=0,2\left(mol\right)\\
pthh:Mg+2HCl\rightarrow MgCl_2+H_2\)
0,2 0,4 0,2 0,2
\(m_{HCl}=0,4.36,5=14,6g\\
V_{H_2}=0,2.22,4=4,48l\\
m\text{dd}=4,8+200-0,4=204,4g\\
C\%=\dfrac{0,2.136}{204,4}.100\%=13,3\%\)

\(a.n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\\ n_{HCl}=\dfrac{17,8\%.200}{36,5}=\dfrac{356}{365}\left(mol\right)\\ Zn+2HCl\rightarrow ZnCl_2+H_2\\ Vì:\dfrac{0,1}{1}< \dfrac{\dfrac{356}{365}}{2}\\ \Rightarrow Znhết,HCldư\\ n_{HCl\left(dùng\right)}=0,1.2=0,2\left(mol\right)\\ m_{HCl\left(dùng\right)}=0,2.36,5=7,3\left(g\right)\\ b.n_{H_2}=n_{ZnCl_2}=n_{Zn}=0,1\left(mol\right)\\ V_{H_2\left(đktc\right)}=0,1.22,4=2,24\left(l\right)\\ c.n_{HCl\left(Dư\right)}=\dfrac{356}{365}-0,2=\dfrac{283}{365}\left(mol\right)\\ C\%_{ddZnCl_2}=\dfrac{0,1.136}{6,5+200}.100\approx6,586\%\)
\(C\%_{ddHCl\left(dư\right)}=\dfrac{\dfrac{283}{365}.36,5}{6,5+200}.100\approx13,705\%\)

Coi như p/ứ vừa đủ
PTHH: \(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\uparrow\)
Ta có: \(n_{Zn}=\dfrac{19,5}{65}=0,3 \left(mol\right)=n_{H_2SO_4}=n_{ZnSO_4}=n_{H_2}\)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{H_2SO_4}=\dfrac{0,3\cdot98}{200}=14,7\%\\V_{H_2}=0,3\cdot22,4=6,72\left(l\right)\\m_{H_2}=0,3\cdot2=0,6\left(g\right)\\m_{ZnSO_4}=0,3\cdot161=48,3\left(g\right)\end{matrix}\right.\)
Mặt khác: \(m_{dd\left(sau.p/ứ\right)}=m_{Zn}+m_{ddH_2SO_4}-m_{H_2}=218,9\left(g\right)\)
\(\Rightarrow C\%_{ZnSO_4}=\dfrac{48,3}{218,9}\cdot100\%\approx22,06\%\)

a, Zn + 2HCl ---> ZnCl2 + H2
b, nZn=\(\dfrac{13}{65}=0,2mol\)
Ta có: 1 mol Zn ---> 1 mol H2
nên 0,2 mol Zn ---> 0,2 mol H2
VH2=0,2.22,4=4,48 mol

PT: \(Al_2O_3+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2O\)
a, Ta có: \(n_{Al_2O_3}=\dfrac{10,2}{102}=0,1\left(mol\right)\)
\(m_{H_2SO_4}=\dfrac{200.20}{100}=40\left(g\right)\Rightarrow n_{H_2SO_4}=\dfrac{40}{98}=\dfrac{20}{49}\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,1}{1}< \dfrac{\dfrac{20}{49}}{3}\), ta được H2SO4 dư.
Theo PT: \(n_{H_2SO_4\left(pư\right)}=3n_{Al_2O_3}=0,3\left(mol\right)\)
\(\Rightarrow n_{H_2SO_4\left(dư\right)}=\dfrac{53}{490}\left(mol\right)\)
\(\Rightarrow m_{H_2SO_4\left(dư\right)}=\dfrac{53}{490}.98=10,6\left(g\right)\)
b, Theo PT: \(n_{Al_2\left(SO_4\right)_3}=n_{Al_2O_3}=0,1\left(mol\right)\)
\(\Rightarrow m_{Al_2\left(SO_4\right)_3}=0,1.342=34,2\left(g\right)\)
c, Ta có: m dd sau pư = mAl2O3 + m dd H2SO4 = 210,2 (g)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{H_2SO_4\left(dư\right)}=\dfrac{10,6}{210,2}.100\%\approx5,04\%\\C\%_{Al_2\left(SO_4\right)_3}=\dfrac{34,2}{210,2}.100\%\approx16,3\%\end{matrix}\right.\)
Bạn tham khảo nhé!

\(n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\)
Pt : \(Zn+2HCl\rightarrow ZnCl_2+H_2|\)
1 2 1 1
0,2 0,2 0,2
\(n_{H2}=\dfrac{0,2.1}{1}=0,2\left(mol\right)\)
\(V_{H2\left(dktc\right)}=0,2.22,4=4,48\left(l\right)\)
\(n_{ZnCl2}=\dfrac{0,2.1}{1}=0,2\left(mol\right)\)
⇒ \(m_{ZnCl2}=0,2.136=27,2\left(g\right)\)
Chúc bạn học tốt
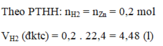

lập pthh của pư
zn+2hcl→zncl2 +h2
1mo 2mol 1mol 1mol
0,2mol 0,2mol 0,2mol
số mol zn
nzn=\(\frac{13}{65}\)=0,2 mol
thể tích khí H2
VH2 = 0,2 . 22,4 =4,48 lít
khối lượng zncl2
mzncl2= 0,2. 136=2,72 gam
nồng độ % dd zncl2
c% zncl2=\(\frac{mct}{mdd}\). 100%=\(\frac{2,72}{200}\). 100%=1,36%