Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{H_2}=\dfrac{3,36}{22,4}=0,,15(mol)\\ Fe+H_2SO_4\to FeSO_4+H_2\\ \Rightarrow n_{Fe}=n_{H_2SO_4}=0,15(mol)\\ a,m_{Fe}=0,15.56=8,4(g)\\ b,C_{M_{H_2SO_4}}=\dfrac{0,15}{0,8}=0,1875M\)

Câu 3 :
\(n_{H2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
Pt : \(Fe+H_2SO_4\rightarrow FeSO_4+H_2|\)
1 1 1 1
0,15 0,15 0,15
a) \(n_{Fe}=\dfrac{0,15.1}{1}=0,15\left(mol\right)\)
⇒ \(m_{Fe}=0,15.56=8,4\left(g\right)\)
b) \(n_{H2SO4}=\dfrac{0,15.1}{1}=0,15\left(mol\right)\)
800ml = 0,8l
\(C_{M_{ddH2SO4}}=\dfrac{0,15}{0,8}=0,1875\left(M\right)\)
Chúc bạn học tốt

\(Fe+H_2SO_4\rightarrow FeSO_4+H_2\)
a) \(n_{Fe}=n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\\ \Rightarrow m_{Fe}=0,15.56=8,4\left(g\right)\)
b) \(n_{H_2SO_4}=n_{HCl}=0,15\left(mol\right)\\ \Rightarrow CM_{H_2SO_4}=\dfrac{0,15}{0,8}=0,1875M\)

a, PT: \(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\)
Ta có: \(n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
Theo PT: \(n_{Zn}=n_{H_2SO_4}=n_{ZnCl_2}=n_{H_2}=0,15\left(mol\right)\)
b, mZn = 0,15.65 = 9,75 (g)
c, CM (H2SO4) = 0,15/0,05 = 3 M
d, mZnSO4 = 0,15.161 = 24,15 (g)
Bạn tham khảo nhé!

a) PTHH: Zn + H2SO4 -> ZnSO4 + H2
nH2= 0,15(mol)
=> nZn=nH2SO4=nZnSO4=nH2=0,15(mol)
b) mZn=0,15.65=9,75(g)
c) CMddH2SO4= 0,15/ 0,05=3(M)
d) mZnSO4= 161. 0,15=24,15(g)

\(Fe+H_2SO_4 \to FeSO_4+H_2\\ n_{H_2}=0,15(mol)\\ a/\\ n_{Fe}=n_{H_2}=0,15(mol)\\ m_{Fe}=0,15.56=8,4(g)\\ b/\\ n_{H_2SO_4}=n_{H_2}=0,15(mol)\\ CM_{H_2SO_4}=\dfrac{0,15}{2}=0,75M c/\\ n_{FeSO_4}=n_{H_2}=0,15(mol)\\ CM_{FeSO_4}=\dfrac{0,15}{0,2}=0,75M\\\)
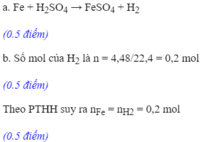

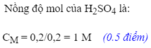
a.Fe+H2SO4---->FeSO4+h2
b. nH2=0,15 mol
Fe+H2SO4---->FeSO4+h2
0,15<--0,15<---------------0,15
mFe=0,15.56=8,4g
c. n h2so4=0,15 mol
C M=0,15/0,6=0,25 M
pt Fe+H2SO4---->FeSO4+H2
nH2=3,36/22,4=0,15 mol
theo pt nFe=nH2=0,15 mol =>mFe=0,15*56=8,4 g
theo pt n H2SO4=nH2=0,15 MOL =>Cm H2SO4=0,15:0,6=0,25M