Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Gọi \(\left\{{}\begin{matrix}n_{Ag}=a\left(mol\right)\\n_{FeO}=b\left(mol\right)\end{matrix}\right.\)
\(n_{SO_2}=\dfrac{1,344}{22,4}=0,6\left(mol\right)\)
PTHH:
\(2Ag+2H_2SO_4\rightarrow Ag_2SO_4+SO_2\uparrow+2H_2O\)
a a \(\dfrac{a}{2}\) \(\dfrac{a}{2}\)
\(2FeO+4H_2SO_4\rightarrow Fe_2\left(SO_4\right)_3+SO_2\uparrow+4H_2O\)
b 2b \(\dfrac{b}{2}\) \(\dfrac{b}{2}\)
Hệ pt
\(\left\{{}\begin{matrix}108a+72b=11,52\\\dfrac{a}{2}+\dfrac{b}{2}=0,06\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,08\left(mol\right)\\b=0,04\left(mol\right)\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}m_{Ag}=0,08.108=8,64\left(g\right)\\m_{FeO}=0,04.72=2,88\left(g\right)\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}\%m_{Ag}=\dfrac{8,64}{11,52}=75\%\\\%m_{FeO}=100\%-75\%=25\%\end{matrix}\right.\)
b, \(\rightarrow n_{H_2SO_4}=0,08+0,4.2=0,16\left(mol\right)\\ \rightarrow C_{MddH_2SO_4}=\dfrac{0,16}{0,8}=0,2M\)
c, \(n_{NaOH}=1,25.0,5=0,625\left(mol\right)\)
PTHH:
\(6NaOH+Fe_2\left(SO_4\right)_3\rightarrow2Fe\left(OH\right)_3+3Na_2SO_4\)
LTL: \(\dfrac{0,625}{6}>\dfrac{0,04}{2}\) => NaOH dư
Theo pthh:
\(\left\{{}\begin{matrix}n_{NaOH\left(pư\right)}=6n_{Fe_2\left(SO_4\right)_3}=6.0,04=0,24\left(mol\right)\\n_{Na_2SO_4}=3n_{Fe_2\left(SO_4\right)_3}=3.0,04=0,12\left(mol\right)\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}C_{MddNaOH\left(dư\right)}=\dfrac{0,24}{0,5}=0,48M\\C_{MddNa_2SO_4}=\dfrac{0,12}{0,5}=0,24M\end{matrix}\right.\)

Câu 1 :
\(n_{H_2SO_4}=0.2\cdot0.1=0.02\left(mol\right)\)
\(n_{KOH}=0.3\cdot0.1=0.03\left(mol\right)\)
\(2KOH+H_2SO_4\rightarrow K_2SO_4+2H_2O\)
\(2..............1\)
\(0.03............0.02\)
Lập tỉ lệ : \(\dfrac{0.03}{2}< \dfrac{0.02}{1}\) \(\Rightarrow H_2SO_4dư\)
\(n_{K_2SO_4}=\dfrac{0.03}{2}=0.015\left(mol\right)\)
\(n_{H_2SO_{4\left(dư\right)}}=0.02-0.015=0.005\left(mol\right)\)
\(V_{ddX}=0.2+0.3=0.5\left(l\right)\)
\(C_{M_{K_2SO_4}}=\dfrac{0.015}{0.5}=0.03\left(M\right)\)
\(C_{M_{H_2SO_4\left(dư\right)}}=\dfrac{0.005}{0.5}=0.01\left(M\right)\)
Câu 2 :
\(n_{H_2}=\dfrac{0.672}{22.4}=0.03\left(mol\right)\)
Hai kim loại ở 2 chu kỳ liên kết thuộc nhóm IA => Đặt CT chung là : M
\(M+H_2O\rightarrow MOH+\dfrac{1}{2}H_2\)
\(0.06............................0.03\)
\(M_M=\dfrac{0.6}{0.06}=10\)\(\Rightarrow9< 10< 23\)
Hai kim loại là : Li và Na
\(n_{Li}=a\left(mol\right),n_{Na}=b\left(mol\right)\)
\(m_{hh}=9a+23b=0.6\left(g\right)\left(1\right)\)
\(n_{H_2}=0.5a+0.5b=0.03\left(mol\right)\left(2\right)\)
\(\left(1\right),\left(2\right):a=0.056,b=0.004\)
\(\%m_{Li}=\dfrac{0.056\cdot9}{0.6}\cdot100\%=84\%\)
\(\%m_{Na}=100-84=16\%\)

Số mol SO2 và NaOH lần lượt là 0,2 và 0,25.
1 < OH-/SO2=1,25 < 2 ⇒ Dung dịch X chứa hai muối Na2SO3 và NaHSO3.
\(n_{Na_2SO_3}=0,25-0,2=0,05\left(mol\right)\) ⇒ \(n_{NaHSO_3}=0,2-0,05=0,15\left(mol\right)\).
1. Khối lượng muối có trong X:
m=0,05.126+0,15.104=21,9 (g).
2. Nồng độ mol/l các chất trong X:
\(C_{M\left(Na_2SO_3\right)}\)=0,05/0,2=0,25 (mol/l).
\(C_{M\left(NaHSO_3\right)}\)=0,15/0,2=0,75 (mol/l).
3. Khối lượng kết tủa BaSO3 là:
m'=0,2.217=43,4 (g).

Chọn D
Gọi N 2 C O 3 (x mol) và N a H C O 3 ( y mol)
Nhỏ từ từ HCl vào hỗn hợp xảy ra pư theo thứ tự:
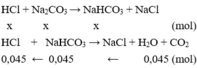


1)
- TN1:
\(n_{AgCl}=\dfrac{35,875}{143,5}=0,25\left(mol\right)\)
PTHH: AgNO3 + HCl --> AgCl + HNO3
0,25<--0,25
TN2:
nNaOH = 0,5.0,3 = 0,15 (mol)
PTHH: NaOH + HCl --> NaCl + H2O
0,15--->0,15
\(n_{HCl\left(dd.C\right)}=0,25+0,15\) = 0,4 (mol)
=> \(C_{M\left(dd.C\right)}=\dfrac{0,4}{2}=0,2M\)
2)
Có \(\left\{{}\begin{matrix}C_{M\left(A\right)}=\dfrac{0,25}{V}M\\C_{M\left(B\right)}=\dfrac{0,15}{V^,}M\end{matrix}\right.\)
nHCl(A) = \(\dfrac{0,025}{V}\left(mol\right)\)
PTHH: Fe + 2HCl --> FeCl2 + H2
\(\dfrac{0,025}{V}\)------>\(\dfrac{0,0125}{V}\)
nHCl(B) = \(\dfrac{0,015}{V^,}\) (mol)
PTHH: Fe + 2HCl --> FeCl2 + H2
\(\dfrac{0,015}{V^,}\)-------->\(\dfrac{0,0075}{V^,}\)
TH1: \(\dfrac{0,0125}{V}=\dfrac{0,0075}{V^,}+0,02\)
Mà V + V' = 2 (l)
=> \(\left[{}\begin{matrix}V=1,5;V^,=0,5\left(KTM\right)\\V=0,5;V^,=1,5\left(TM\right)\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}C_{M\left(A\right)}=\dfrac{0,25}{0,5}=0,5M\\C_{M\left(B\right)}=\dfrac{0,15}{1,5}=0,1M\end{matrix}\right.\)
TH2: \(\dfrac{0,0125}{V}+0,02=\dfrac{0,0075}{V^,}\)
=> \(\left[{}\begin{matrix}V=\dfrac{1+\sqrt{6}}{2};V^,=\dfrac{3-\sqrt{6}}{2}\\V=\dfrac{1-\sqrt{6}}{2}\left(L\right)\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}C_{M\left(A\right)}=\dfrac{0,25}{\dfrac{1+\sqrt{6}}{2}}=\dfrac{-1+\sqrt{6}}{10}M\\C_{M\left(B\right)}=\dfrac{0,15}{\dfrac{3-\sqrt{6}}{2}}=\dfrac{3+\sqrt{6}}{10}M\end{matrix}\right.\)

a) \(n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
PTHH: R + 2HCl --> RCl2 + H2
____0,15<-----------------0,15
=> \(M_R=\dfrac{3,6}{0,15}=24\left(Mg\right)\)
b)
PTHH: Mg + 2HCl --> MgCl2 + H2
__________0,3<-----0,15<---0,15
=> \(V=\dfrac{0,3}{2}=0,15\left(l\right)=150\left(ml\right)\)
\(C_{M\left(MgCl_2\right)}=\dfrac{0,15}{0,15}=1M\)

\(a.n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\\ PTHH:R+2HCl\rightarrow RCl_2+H_2\\ \Rightarrow n_R=n_{H_2}=0,15\left(mol\right)\\ \Rightarrow M_R=\dfrac{m_R}{n_R}=\dfrac{3,6}{0,15}=24\\ \)
\(\Rightarrow R\) là \(Magie\left(Mg\right)\)
\(b.n_{HCl}=2.n_{H_2}=2.0,15=0,3\left(mol\right)\\ \Rightarrow V_{ddHCl}=\dfrac{n_{HCl}}{C_M}=\dfrac{0,3}{2}=0,15\left(l\right)=150ml\)
\(n_{MgCl_2}=n_{Mg}=0,15\left(mol\right)\\ \Rightarrow C_{M_{ddMgCl_2}}=\dfrac{n_{MgCl_2}}{V}=\dfrac{0,15}{0,15}=1\left(M\right)\)
