Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(CH_4+2O_2\underrightarrow{^{to}}CO_2+2H_2O\\ n_{H_2O}=\dfrac{3,6}{18}=0,2\left(mol\right)\\ n_{CH_4\left(LT\right)}=\dfrac{0,2}{2}=0,1\left(mol\right)\\ n_{CH_4\left(TT\right)}=0,1:\left(100\%-10\%\right)=\dfrac{1}{9}\left(mol\right)\\ V_{CH_4\left(TT\right)}=\dfrac{1}{9}.22,4\approx2,489\left(l\right)\)

\(n_{KClO_3}=\dfrac{24,5}{122,5}=0,2\left(mol\right)\\ 2KClO_3\underrightarrow{^{to}}2KCl+3O_2\\ n_{O_2\left(LT\right)}=\dfrac{3}{2}.0,2=0,3\left(mol\right)\\ Vì:H=90\%\Rightarrow n_{O_2\left(TT\right)}=90\%.0,3=0,27\left(mol\right)\\ V_{O_2\left(đktc,thực.tế\right)}=0,27.22,4=6,048\left(l\right)\)

a)
\(n_{KClO_3}=\dfrac{24.5}{122.5}=0.2\left(mol\right)\)
\(2KClO_3\underrightarrow{^{^{t^0}}}2KCl+3O_2\)
\(n_{O_2}=\dfrac{3}{2}\cdot0.2=0.3\left(mol\right)\)
\(V_{O_2}=0.3\cdot22.4=6.72\left(l\right)\)
\(n_{O_2}=\dfrac{2.24}{22.4}=0.1\left(mol\right)\)
\(2KMnO_4\underrightarrow{^{^{t^0}}}K_2MnO_4+MnO_2+O_2\)
\(0.2...............................................0.1\)
\(n_{KMnO_4\left(bđ\right)}=\dfrac{0.2}{90\%}=\dfrac{2}{9}\left(mol\right)\)
\(m_{KMnO_4}=\dfrac{2}{9}\cdot158=35.11\left(g\right)\)

2KClO3-to>2KCl+3O2
0,06-----------------0,09 mol
n O2=2,016\22,4=0,09 mol
=>H =0,06.122,5\12,25 .100=60%
\(n_{O_2\left(TT\right)}=\dfrac{2,016}{22,4}=0,09\left(mol\right)\\ n_{KClO_3}=\dfrac{12,25}{122,5}=0,1\left(mol\right)\\ 2KClO_3\underrightarrow{^{to}}2KCl+3O_2\\ n_{O_2\left(LT\right)}=\dfrac{3}{2}.0,1=0,15\left(mol\right)\\ \Rightarrow H=\dfrac{0,09}{0,15}.100=60\%\)

2KMnO4-to>K2MnO4+MnO2+O2
1,2-------------------------------------0,6 mol
n O2=13,44\22,4=0,6 mol
H =75%
=>m KMnO4 tt= 1,2.158 .100\75=252,8g
\(n_{O_2}=\dfrac{13,44}{22,4}=0,6\left(mol\right)\)
PTHH: 2KMnO4 ---to→ K2MnO4 + MnO2 + O2
Mol: 1,2 0,6
\(m_{KMnO_4\left(lt\right)}=1,2.158=189,6\left(g\right)\Rightarrow m_{KMnO_4\left(tt\right)}=\dfrac{189,6}{75}.100=252,8\left(g\right)\)

\(n_{O_2}=\dfrac{0,672}{22,4}=0,03\left(mol\right)\\ n_{KMnO_4}=\dfrac{15,8}{158}=0,1\left(mol\right)\\ 2KMnO_4\underrightarrow{^{to}}K_2MnO_4+MnO_2+O_2\\ n_{O_2\left(LT\right)}=\dfrac{0,1}{2}=0,05\left(mol\right)\\ H=\dfrac{0,03}{0,05}.100=60\%\)
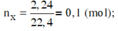
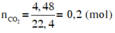
2KClO3-to>2KCl+3O2
0,08--------------------0,12 mol
n O2=2,688\22,4=0,12 mol
H=20%
=>m KClO3tt=0,08.122,5.100\20=49g
\(n_{O_2}=\dfrac{2,688}{22,4}=0,12\left(mol\right)\\ 2KClO_3\underrightarrow{^{to}}2KCl+3O_2\\ n_{KClO_3\left(LT\right)}=\dfrac{2}{3}.0,12=0,08\left(mol\right)\\ Hao.hụt.80\%.Nên:n_{KClO_3\left(TT\right)}=0,08:\left(100\%-20\%\right)=0,1\left(mol\right)\\\Rightarrow m=m_{KClO_3\left(TT\right)}=122,5.0,1=12,25\left(g\right)\)