Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

nFe=0,2(mol)
a) PTHH: Fe2O3 + 3 H2 -to-> 2 Fe + 3 H2O
0,1_____________0,3____0,2(mol)
b) mFe2O3=160.0,1=16(g)
c) V(H2,đktc)=0,3.22,4=6,72(l)

a, \(Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
b, \(n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\)
Theo PT: \(n_{Fe_2O_3}=\dfrac{1}{2}n_{Fe}=0,1\left(mol\right)\Rightarrow m_{Fe_2O_3}=0,1.160=16\left(g\right)\)
c, Theo PT: \(n_{H_2}=\dfrac{3}{2}n_{Fe}=0,3\left(mol\right)\)
\(\Rightarrow V_{H_2}=0,3.22,4=6,72\left(l\right)\)

\(n_{Fe_2O_3=}=\dfrac{24}{160}=0,15mol\)
\(Fe_2O_3+3H_2\rightarrow2Fe+3H_2O\)
0,15 0,45 0,3 0,45
\(V_{H_2}=0,45\cdot224,=10,08l\)
\(m_{Fe}=0,3\cdot56=16,8g\)

a, \(Fe_3O_4+4H_2\underrightarrow{t^o}3Fe+4H_2O\)
b, \(n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\)
Theo PT: \(n_{Fe_3O_4}=\dfrac{1}{3}n_{Fe}=\dfrac{1}{15}\left(mol\right)\Rightarrow m_{Fe_3O_4}=\dfrac{1}{15}.232=\dfrac{232}{15}\left(g\right)\)
c, \(n_{H_2}=\dfrac{4}{3}n_{Fe}=\dfrac{4}{15}\left(mol\right)\Rightarrow V_{H_2}=\dfrac{4}{15}.22,4=\dfrac{448}{75}\left(l\right)\)
d, \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(n_{Zn}=n_{H_2}=\dfrac{4}{15}\left(mol\right)\Rightarrow m_{Zn}=\dfrac{4}{15}.65=\dfrac{52}{3}\left(g\right)\)
\(n_{HCl}=2n_{H_2}=\dfrac{8}{15}\left(mol\right)\Rightarrow m_{HCl}=\dfrac{8}{15}.36,5=\dfrac{292}{15}\left(g\right)\)

a, \(Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
b, \(n_{Fe}=\dfrac{22,4}{56}=0,4\left(mol\right)\)
Theo PT: \(n_{Fe_2O_3}=\dfrac{1}{2}n_{Fe}=0,2\left(mol\right)\Rightarrow m_{Fe_2O_3}=0,2.160=32\left(g\right)\)
c, \(n_{H_2}=\dfrac{3}{2}n_{Fe}=0,6\left(mol\right)\Rightarrow V_{H_2}=0,6.22,4=13,44\left(l\right)\)
d, \(2H_2+O_2\underrightarrow{t^o}2H_2O\)
Theo PT: \(n_{O_2}=\dfrac{1}{2}n_{H_2}=0,3\left(mol\right)\Rightarrow V_{O_2}=0,3.22,4=6,72\left(l\right)\)
\(\Rightarrow V_{kk}=\dfrac{V_{O_2}}{20\%}=33,6\left(l\right)\)
a)
$Fe_2O_3 + 3H_2 \xrightarrow{t^o} 2Fe + 3H_2O$
b) $n_{Fe} = \dfrac{22,4}{56} = 0,4(mol)$
Theo PTHH : $n_{Fe_2O_3} = \dfrac{1}{2}n_{Fe} = 0,2(mol)$
$m_{Fe_2O_3} = 0,2.160 = 32(gam)$
c) $n_{H_2} = \dfrac{3}{2}n_{Fe} = 0,6(mol)$
$V_{H_2} = 0,6.22,4 = 13,44(lít)$
d) $2H_2 + O_2 \xrightarrow{t^o} 2H_2O$
$V_{O_2} = \dfrac{1}{2}V_{H_2} = 6,72(lít)$
$V_{kk} = 6,72 : 20\% = 33,6(lít)$

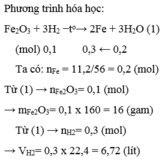
\(n_{Fe}=\dfrac{m_{Fe}}{M_{Fe}}=\dfrac{11,2}{56}=0,2mol\)
\(Fe_2O_3+3H_2\rightarrow2Fe+3H_2O\)
1 3 2 3 ( mol )
0,1 0,3 0,2 ( mol )
\(m_{Fe_2O_3}=n_{Fe_2O_3}.M_{Fe_2O_3}=0,1.160=16g\)
\(V_{H_2}=n_{H_2}.22,4=0,3.22,4=6,72l\)

2)
nH2 = 6.72/22.4 = 0.3 (mol)
Fe2O3 + 3H2 -to-> 2Fe + 3H2O
0.1______0.3______0.2
mFe2O3 = 0.1*160 = 16 (g)
mFe = 0.2*56 = 11.2 (g)
3)
nFe3O4 = 11.6/232 = 0.05 (mol)
3Fe + 2O2 -to-> Fe3O4
0.15___0.1______0.05
mFe = 0.15*56 = 8.4 (g)
VO2 = 0.1*22.4 = 2.24 (l)
2KClO3 -to-> 2KCl + 3O2
1/15______________0.1
mKClO3 = 1/15 * 122.5 = 8.167 (g)
a)
3H2 + Fe2O3 --to--> 2Fe + 3H2O
b) nH2 = 6,72/22,4 = 0,3 mol
Từ pt => nFe3O4 = 0,1 mol
=> mFe3O4 = 0,1. 232 = 23,2 g

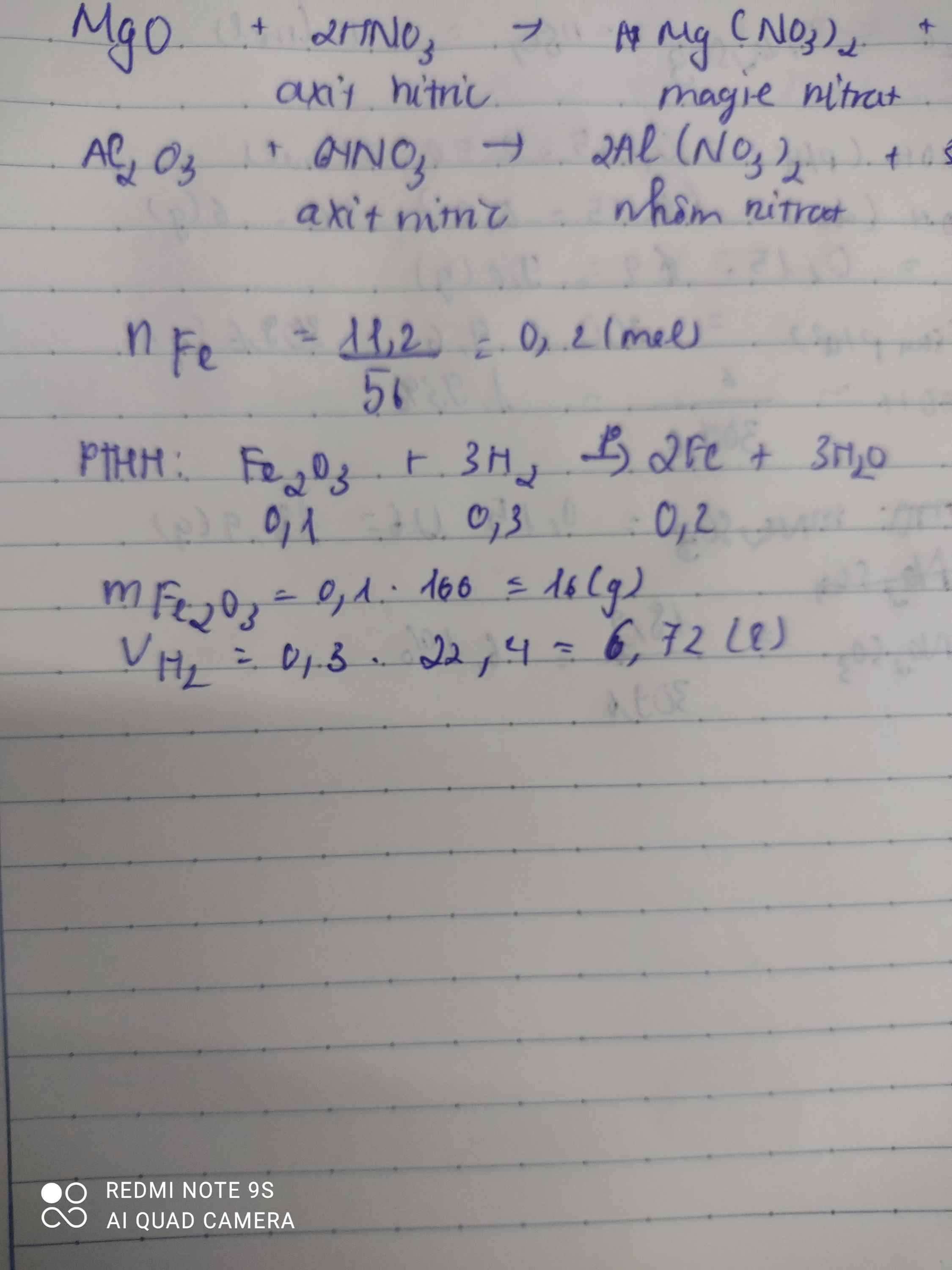
`a)`
`3H_2+Fe_2 O_3` $\xrightarrow{t^o}$ `2Fe + 3H_2 O`
`0,75` `0,25` `0,5` `0,75` `(mol)`
`n_[Fe_2 O_3]=40/160=0,25(mol)`
`b)V_[H_2]=0,75.22,4=16,8(l)`
`c)m_[Fe]=0,5.56=28(g)`
`d)V_[H_2 O]=0,75.22,4=16,8(l)`
a, `3H_2 + Fe_2O_3 -> 2Fe + 3H_2O`.
`=> n_(Fe_2O_3) = (m(Fe_2O_3))/(M_(Fe_2O_3)) = 40/160 = 0,25 mol`.
b,` n_(H_2) = 0,25 xx 3 = 0,75 mol`.
`V_(H_2) = 0,75 xx 22,4 = 16,8l`.
c, `n_(Fe) = 0,25 xx 3 = 0,5 mol`.
`m_(Fe) = n_(Fe) . M_(Fe) = 0,5 xx 56 = 28 g`.
d, `n_(H_2O) = 0,25 xx 3 = 0,75 mol`.
`V_(H_2O) = 0,75 xx 22,4 = 16,8 l`.