Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) \(PTHH:2SO_2+O_2\xrightarrow[V_2O_5]{450^oC}2SO_3\)
\(n_{SO_2}=\dfrac{32}{64}=0,5\left(mol\right)\\ n_{O_2}=\dfrac{10}{32}=0,3125\left(mol\right)\)
Lập tỉ lệ: \(\dfrac{n_{SO_2}}{2}< \dfrac{n_{O_2}}{1}\left(\dfrac{0,5}{2}< 0,3125\right)\)
=> SO2 hết O2 dư
Theo pt: \(n_{O_2\left(pư\right)}=\dfrac{n_{SO_2}.2}{3}=\dfrac{0,5.1}{2}=0,25\left(mol\right)\)
\(n_{O_2\left(dư\right)}=0,3125-0,25=0,0625\left(mol\right)\\ m_{O_2}=0,0625.32=2\left(g\right)\)
c) Theo pt, ta có:\(n_{SO_3}=n_{SO_2}=0,5\left(mol\right)\)
\(m_{SO_3}=0,5.80=40\left(g\right)\)

nS = 1,92/32 = 0,06 (mol)
PTHH: S + O2 -> (t°) SO2
Mol: 0,06 ---> 0,06 ---> 0,06
VSO2 (LT) = 0,06 . 22,4 = 1,344 (l)
VSO2 (TT) = 1,344 . 90% = 1,2096 (l)

\(a) 2Al + 6HCl \to 2AlCl_3 + 3H_2\\ b) n_{H_2} = \dfrac{6,72}{22,4} = 0,3(mol)\\ n_{Al} = \dfrac{2}{3}n_{H_2} = 0,2(mol)\\ m_{Al} = 0,2.27 = 5,4(gam)\\ c) n_{HCl\ pư} = 2n_{H_2} = 0,6(mol)\\ n_{HCl\ đã\ dùng} = \dfrac{0,6}{80\%} = 0,75(mol)\\ m_{dd\ HCl} = \dfrac{0,75.36,5}{54,75\%} = 50(gam)\)

1. a) PTHH: \(2KClO_3=2KCl+3O_2\)
b) Khối lượng \(KClO_3\) thực tế phản ứng:
\(H=\dfrac{m_{tt}}{m_{lt}}.100\%\Rightarrow m_{tt}=\dfrac{m_{lt}.H}{100\%}=11,025\left(g\right)\)
\(n_{KClO_3}=\dfrac{m_{KClO_3}}{M_{KClO_3}}=\dfrac{11,025}{122,5}=0,09\left(mol\right)\)
Theo PTHH: \(n_{O_2}=\dfrac{0,09.3}{2}=0,135\left(mol\right)\)
\(\Rightarrow V_{O_2}=n_{O_2}.22,4=0,135.22,4=3,024\left(l\right)\)
c) \(4Fe+3O_2\xrightarrow[t^o]{}2Fe_2O_3\)
\(n_{Fe}=\dfrac{5,6}{56}=0,1\left(mol\right)\)
Theo PTHH: \(n_{Fe_2O_3}=\dfrac{0,1.2}{4}=0,05\left(mol\right)\)
\(\Rightarrow m_{Fe_2O_3}=n_{Fe_2O_3}.M_{Fe_2O_3}=0,05.160=8\left(g\right)\)
a)Ta có PTHH: 2KClO3 --t---> 2KCl + 3O2 (1)
b) Biết mKClO3 =12,25g => nKClO3 = mKClO3/MKClO3
=12,25/122,5=0,1 (mol)
Theo PT (1) ta có:
no2 =3/2 nKCLO3 =3/2 . 0,1= 0,15(mol)
Vậy VO2 = n . 22,4 = 0,15 . 22,4= 3,36 (L)
c) Ta có PTHH: 4Fe + 3O2 -----> 2Fe2O3 (2)
Biết mFe = 5,6 g => nFe = m/M= 5,6/56=0,1 (mol)
Theo PT (2) ta có :
nFe2O3 = 2/4 nFe = 2/4 .0,1=0,05 (mol)
Vậy mFe2O3 = n . M = 0,05 . 160= 8 (g)

2Al + 3S -> Al2S3 (1)
nAl2S3=\(\dfrac{64}{375}\left(mol\right)\)
nAl=0,4(mol)
Từ 1:
nAl PƯ=2nAl2S3=\(\dfrac{128}{375}\left(mol\right)\)
H=\(\dfrac{128}{375}:0,4.100\%=85,3\%\)

S+O2-to>SO2
0,2--0,2----0,2 mol
n SO2=\(\dfrac{4,48}{22,4}\)=0,2 mol
=>m S=0,2.32=6,4g
=>VO2=0,2.22,4=4,48l

\(n_{O2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
Pt : \(S+O_2\underrightarrow{t^o}SO_2|\)
1 1 1
0,15 0,15 0,15
a) \(n_S=\dfrac{0,15.1}{1}=0,15\left(mol\right)\)
⇒ \(m_S=0,15.32=4,8\left(g\right)\)
b) \(n_{SO2}=\dfrac{0,15.1}{1}=0,15\left(mol\right)\)
\(V_{SO2\left(dktc\right)}=0,15.22,4=3,36\left(l\right)\)
Chúc bạn học tốt

\(n_{Al_2S_3}=\dfrac{25.5}{150}=0.17\left(mol\right)\)
\(n_{Al}=\dfrac{10.8}{27}=0.4\left(mol\right)\)
\(2Al+3S\underrightarrow{^{t^0}}Al_2S_3\)
\(0.34...........0.17\)
\(H\%=\dfrac{0.34}{0.4}\cdot100\%=85\%\)
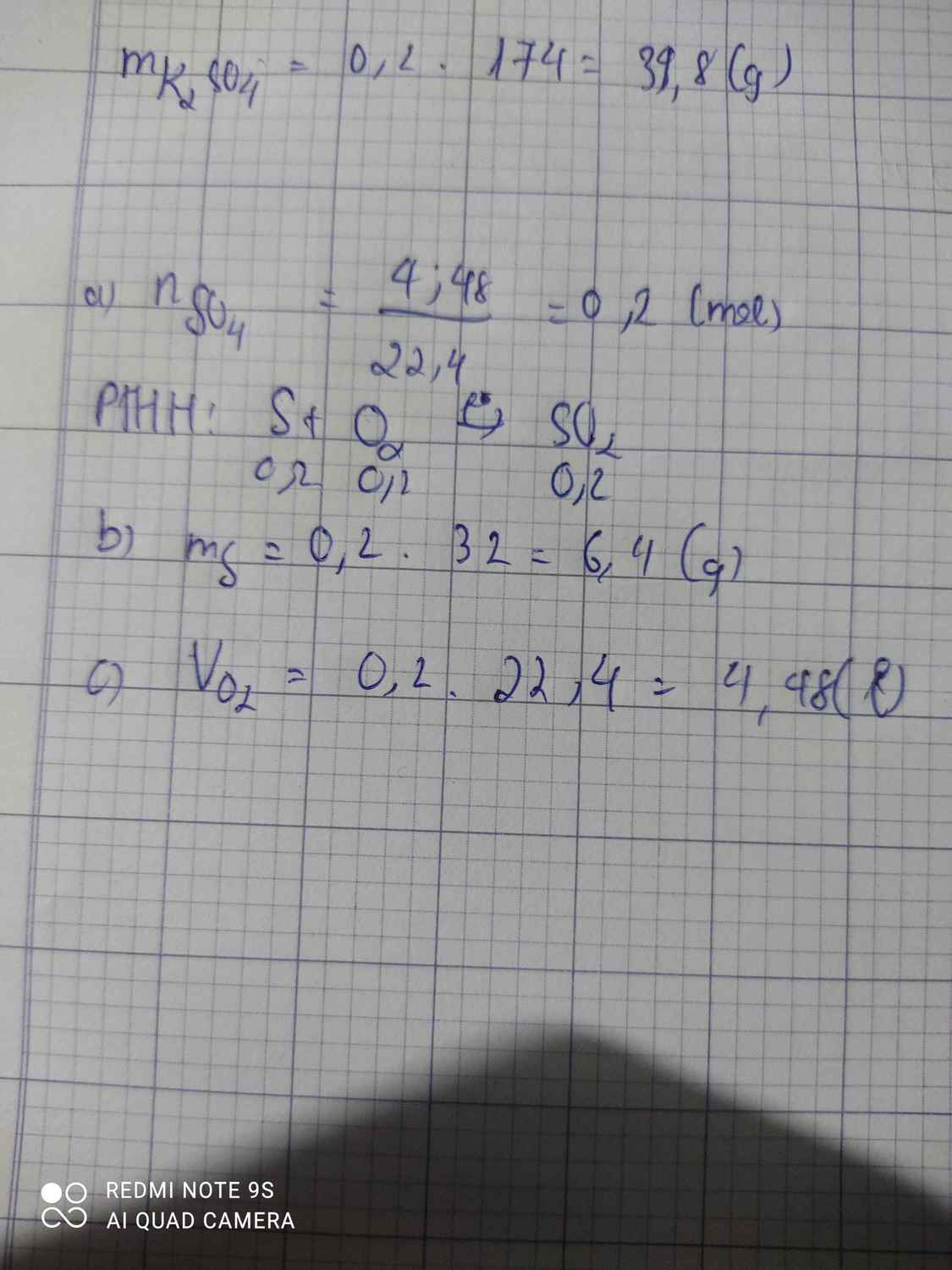

Bài 3 :
a) \(Theo-\text{đ}\text{è}-b\text{ài}-ta-c\text{ó}:\left\{{}\begin{matrix}nCu=\dfrac{51,2}{64}=0,8\left(mol\right)\\nCuO=\dfrac{48}{80}=0,6\left(mol\right)\end{matrix}\right.\)
Ta có PTHH :
2Cu + O2 -t0\(\rightarrow\) 2CuO
Theo PTHH ta có : nCu = \(\dfrac{0,8}{2}mol>nCuO=\dfrac{0,6}{2}mol\) => nCu dư
b) Ta có : H = \(\dfrac{n\left(ch\text{ất}-thi\text{ếu}\right)}{n\left(ch\text{ất}-d\text{ư}\right)}.100\%=\dfrac{0,6}{0,8}.100\%=75\%\)
Vậy.......
Mình c.ơn nhìu lắm lun