Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

1
\(n_{Ba\left(OH\right)_2}=0,25.2=0,5\left(mol\right)\\ \Rightarrow m_{Ba\left(OH\right)_2}=0,5.171=85,5\left(g\right)\)
2
\(n_{FeCl_3}=0,08.0,15=0,012\left(mol\right)\\ \Rightarrow m_{FeCl_3}=0,012.162,5=1,95\left(g\right)\)
3
\(n_{MgSO_4}=4,5.0,8=3,6\left(mol\right)\\ \Rightarrow m_{MgSO_4}=3,6.120=432\left(g\right)\)
4
\(n_{Zn\left(NO_3\right)_2}=0,015.0,4=0,006\left(mol\right)\\ \Rightarrow m_{Zn\left(NO_3\right)_2}=0,006.189=1,134\left(g\right)\)

Câu 3 chưa cho C% em ạ
Câu 4:
\(m_{ct}=12\%.25=3\left(g\right)\)

\(a,m_{rắn}=m_{Cu}=2,7\left(g\right)\\ \Rightarrow m_{\left(Zn,Fe\right)}=12-2,7=9,3\left(g\right)\\ n_{H_2}=0,15\left(mol\right),n_{axit}=2.0,2=0,4\left(mol\right)\\ Đặt:n_{Zn}=a\left(mol\right);n_{Fe}=b\left(mol\right)\left(a,b>0\right)\\ PTHH:Zn+H_2SO_4\rightarrow ZnSO_4+H_2\\ Fe+H_2SO_4\rightarrow FeSO_4+H_2\\ Vì:\dfrac{0,15}{1}< \dfrac{0,4}{1}\Rightarrow axit.dư\\ \Rightarrow\left\{{}\begin{matrix}65+56b=9,3\\a+b=\dfrac{3,36}{22,4}=0,15\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,1\\b=0,05\end{matrix}\right.\\ \Rightarrow\%m_{Cu}=\dfrac{2,7}{12}.100=22,5\%\\ \%m_{Zn}=\dfrac{0,1.65}{12}.100\approx54,167\%\\ \%m_{Fe}=\dfrac{0,05.56}{12}.100\approx23,333\%\)
\(b,ddA:FeCl_2,ZnCl_2,H_2SO_4\left(dư\right)\\ m_{ddH_2SO_4}=200.1,14=228\left(g\right)\\ m_{ddA}=m_{\left(Zn,Fe\right)}+m_{ddH_2SO_4}-m_{H_2}=9,3+228-0,15.2=237\left(g\right)\)
\(C\%_{ddZnCl_2}=\dfrac{136.0,1}{237}.100\approx5,738\%\\ C\%_{ddFeCl_2}=\dfrac{127.0,05}{237}.100\approx2,679\%\\ C\%_{ddH_2SO_4\left(dư\right)}=\dfrac{\left(0,4-0,15\right).98}{237}.100\approx10,338\%\)
Đã sửa lần cuối lúc 20:45

\(a,m_{ddCuSO_4}=\dfrac{24.100}{15}=160\left(g\right)\)
\(b,m_{\left(Al\left(NO_3\right)_3\right)}=0,5.89=44,5\left(g\right)\)
\(m_{ddAl\left(NO_3\right)}=\dfrac{44,5.100}{4}=1112,5\left(g\right)\)
\(c,n_{FeCl_3}=0,8.0,15=0,12\left(mol\right)\)
\(m_{FeCl_3}=0,12.106,5=12,78\left(g\right)\)
Câu c chưa tính được m dung dịch do không có KL riêng

a) nNaCl = 1.0,5 = 0,5 (mol) → mNaCl = 0,5.(23 +35,5) = 29,25 (g)
b) nKNO3 = 2.0,5 = 1 (mol) → mKNO3 = 1.101 = 101 (g)
c) nCaCl2 = 0,1.0,25 = 0,025 (mol) → mCaCl2 = 0,025(40 + 71) = 2,775 (g)
d) nNa2SO4 = 0,3.2 = 0,6 (mol) → mNa2SO4 = 0,6.142 = 85,2 (g)

`PTPƯ: Zn + H_2 SO_4-> ZnSO_4 + H_2↑`
`n_[Zn] = [ 6,5 ] / 65= 0,1 (mol)`
Theo `PTPƯ` có: `n_[ZnSO_4] = n_[Zn] = 0,1 (mol)`
`-> m_[ZnSO_4] = 0,1 . 161 = 16,1 (g)`

a, mchất rắn = mCu = 12,8 (g)
=> mhh (Al, Zn) = 28,5 - 12,8 = 16,7 (g)
\(m_{H_2SO_4}=7,84\%.500=39,2\left(g\right)\\ n_{H_2SO_4}=\dfrac{39,2}{98}=0,4\left(mol\right)\)
Gọi \(\left\{{}\begin{matrix}n_{Al}=a\left(mol\right)\\n_{Zn}=b\left(mol\right)\end{matrix}\right.\left(a,b>0\right)\)
PTHH:
2Al + 3H2SO4 ---> Al2(SO4)3 + 3H2↑
a----->1,5a---------->0,5a-------->1,5a
Zn + H2SO4 ---> ZnSO4 + H2
b---->b------------>b--------->b
mdd (tăng) = mhh (Al, Zn) - mH2 = 27a + 65a - 2.(1,5a - b) = 24a - 63b = 515 - 500 = 15 (g)
=> Hệ pt \(\left\{{}\begin{matrix}27a+65b=15,7\\24a-63b=15\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,1\left(mol\right)\\b=0,2\left(mol\right)\end{matrix}\right.\left(TM\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Cu}=\dfrac{12,8}{28,5}.100\%=44,9\%\\\%m_{Al}=\dfrac{0,1.27}{28,5}.100\%=18,9\%\\\%m_{Zn}=100\%-44,9\%-18,9\%=36,2\%\end{matrix}\right.\)
b, \(n_{H_2SO_{4\left(dư\right)}}=0,4-0,1.1,5-0,2=0,05\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{Al_2\left(SO_4\right)_3}=\dfrac{0,05.342}{515}.100\%=3,32\%\\C\%_{ZnSO_4}=\dfrac{0,2.161}{515}.100\%=6,25\%\\C\%_{H_2SO_{4\left(dư\right)}}=\dfrac{0,05.98}{515}.100\%=0,95\%\end{matrix}\right.\)
anh ơi \(24a+63b=15\) mới đúng chứ anh:)
\(m_{dd\left(tăng\right)}=24a+63b\) nữa:)

a, PT: \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
Gọi: \(\left\{{}\begin{matrix}n_{Zn}=x\left(mol\right)\\n_{Al}=y\left(mol\right)\end{matrix}\right.\) ⇒ 65x + 27y = 17,05 (1)
Ta có: \(n_{H_2}=\dfrac{9,52}{22,4}=0,425\left(mol\right)\)
Theo PT: \(n_{H_2}=n_{Zn}+\dfrac{3}{2}n_{Al}=x+\dfrac{3}{2}y=0,425\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}n_{Zn}=0,2\left(mol\right)\\n_{Al}=0,15\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}m_{Zn}=0,2.65=13\left(g\right)\\m_{Al}=0,15.27=4,05\left(g\right)\end{matrix}\right.\)
b, Theo PT: \(\left\{{}\begin{matrix}n_{ZnCl_2}=n_{Zn}=0,2\left(mol\right)\\n_{AlCl_3}=n_{Al}=0,15\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}C_{M_{ZnCl_2}}=\dfrac{0,2}{0,5}=0,4\left(M\right)\\C_{M_{AlCl_3}}=\dfrac{0,15}{0,5}=0,3\left(M\right)\end{matrix}\right.\)
c, Ta có: m dd HCl = 1,05.500 = 525 (g)
m dd sau pư = mhh + m dd HCl - mH2 = 541,2 (g)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{ZnCl_2}=\dfrac{0,2.136}{541,2}.100\%\approx5,03\%\\C\%_{AlCl_3}=\dfrac{0,15.133,5}{541,2}.100\%\approx3,7\%\end{matrix}\right.\)

Số gam chất tan cần dùng để pha chế các dung dịch:
a) nNaCl = CM .V = 2,5.0,9 = 2,25 (mol)
→ mNaCl = 2,25.(23 + 35,5) = 131,625 (g)
b) 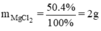
c) nMgSO4 = 0,1.0,25 = 0,025 (mol)
→ mMgSO4 = 0,025.(24 + 64 + 32) = 3 (g)