Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

3. PTHH: (1) Fe + 2 HCl -> FeCl2 + H2
x______________2x______x___x(mol)
(2) 2 Al + 6 HCl -> 2 AlCl3 + 3 H2
y__________3y_____y___________1,5y(mol)
nH2= 5,6/22,4 =0,25 (mol)
Ta có: \(\left\{{}\begin{matrix}56x+27y=8,3\\x+1,5y=0,25\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=0,1\\y=0,1\end{matrix}\right.\)
=> mFe= 0,1.56= 5,6(g) => %mFe=\(\frac{5,6}{8,3}.100\approx67,47\%\\ =>\%mAl\approx32,53\%\)

Gọi số mol Al, Fe là a, b (mol)
=> 27a + 56b = 11,1 (1)
\(n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
PTHH: 2Al + 6HCl --> 2AlCl3 + 3H2
a----------------------->1,5a
Fe + 2HCl --> FeCl2 + H2
b------------------------>b
=> 1,5a + b = 0,3 (2)
(1)(2) => a = 0,1; b = 0,15
=> \(\left\{{}\begin{matrix}\%m_{Al}=\dfrac{0,1.27}{11,1}.100\%=24,32\%\\\%m_{Fe}=\dfrac{0,15.56}{11,1}.100\%=75,68\end{matrix}\right.\)

\(n_{H_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\\ PTHH:2Al+6HCl\rightarrow2AlCl_3+3H_2\\ Fe+2HCl\rightarrow FeCl_2+3H_2\\ Đặt:n_{Al}=a\left(mol\right);n_{Fe}=b\left(mol\right)\left(a,b>0\right)\\ \Rightarrow\left\{{}\begin{matrix}27a+56b=11\\1,5a+b=0,4\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,2\\b=0,1\end{matrix}\right.\\ a,n_{HCl}=2.n_{H_2}=2.0,4=0,8\left(mol\right)\\ \Rightarrow V_{ddHCl}=\dfrac{0,8}{8}=0,1\left(l\right)\\ b,FeCl_2+2AgNO_3\rightarrow Fe\left(NO_3\right)_2+2AgCl\downarrow\\ AlCl_3+3AgNO_3\rightarrow Al\left(NO_3\right)_3+3AgCl\downarrow\\ n_{AgCl}=n_{AgNO_3}=3.n_{AlCl_3}+2.n_{FeCl_2}=3.a+2.b=3.0,2+2.0,1=0,8\left(mol\right)\\ \Rightarrow a=\dfrac{170.0,8}{250}.100=54,4\%\\ b=m_{\downarrow}=m_{AgCl}=0,8.143,5=114,8\left(g\right)\)

\(n_{H_2}=\dfrac{V_{H_2}}{22,4}=\dfrac{20,16}{22,4}=0,9mol\)
Gọi \(\left\{{}\begin{matrix}n_{Al}=x\\n_{Mg}=y\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{Al}=27x\\m_{Mg}=24y\end{matrix}\right.\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
x \(\dfrac{3}{2}x\) ( mol )
\(Mg+2HCl\rightarrow MgCl_2+H_2\)
y y ( mol )
Ta có:
\(\left\{{}\begin{matrix}27x+24y=19,8\\\dfrac{3}{2}x+y=0,9\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}x=0,2\\y=0,6\end{matrix}\right.\)
\(\Rightarrow m_{Al}=0,2.27=5,4g\)
\(\Rightarrow m_{Mg}=0,6.24=14,4g\)
\(\%m_{Al}=\dfrac{5,4}{19,8}.100=27,27\%\)
\(\%m_{Mg}=100\%-27,27\%=72,73\%\)

Câu 1:
Gọi số mol Al là x; Zn là y
\(\rightarrow27x+65y=18,4\)
\(Al+3HCl\rightarrow AlCl_3+\frac{3}{2}H_2\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(\rightarrow n_{H2}=1,5n_{Al}+n_{Zn}=1,5x+y=\frac{1}{2}=0,5\left(mol\right)\)
Giải được: \(x=y=0,2\)
\(\Rightarrow m_{Al}=27x=5,4\left(g\right)\Rightarrow\%m_{Al}=\frac{5,4}{18,4}=29,3\%\Rightarrow\%m_{Zn}=70,7\%\)Câu 2:
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(FeO+2HCl\rightarrow FeCl_2+H_2\)
Ta có: \(n_{H2}=n_{Fe}=\frac{2,24}{22,4}=0,1\left(mol\right)\)
Muối thu được là FeCl2
\(\rightarrow n_{FeCl2}=\frac{38,1}{56+35,5.2}=0,3\left(mol\right)\)
Ta có: \(n_{FeCl2}=n_{Fe}+n_{FeO}\rightarrow n_{FeO}=0,2\left(mol\right)\)
\(\Rightarrow m_{Fe}=0,1.56=5,6\left(g\right);m_{FeO}=0,2.\left(56+16\right)=14,4\left(g\right)\)
Câu 3 :
Cu không tác dụng với HCl, chỉ có Zn phản ứng.
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
Ta có: \(n_{H2}=\frac{4,48}{22,4}=0,2\left(mol\right)\)
Theo phản ứng: \(n_{Zn}=n_{H2}=0,2\left(mol\right)\rightarrow m_{Zn}=0,2.65=13\left(g\right)\)
\(\rightarrow\%m_{Zn}=\frac{13}{20}=65\%\rightarrow\%m_{Cu}=35\%\)
Ta có: \(n_{HCl}=2n_{H2}=0,2.2=0,4\left(mol\right)\)
\(\Rightarrow V_{HCl}=\frac{0,4}{2}=0,2\left(l\right)\)
Câu 4:
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(Al+3HCl\rightarrow AlCl_3+\frac{3}{2}H_2\)
Gọi số mol Fe là x; Al là y
\(\rightarrow56x+27y=22\)
Ta có: \(n_{H2}=n_{Fe}=1,5n_{Al}=x+1,5y=\frac{17,92}{22,4}=0,8\left(mol\right)\)
Giải được: \(\rightarrow\left\{{}\begin{matrix}x=0,2\\y=0,4\end{matrix}\right.\)
\(\Rightarrow m_{Fe}=0,2.56=11,2\left(g\right)\)
\(\rightarrow\%m_{Fe}=\frac{11,2}{22}=50,9\%\rightarrow\%m_{Al}=49,1\%\)
Ta có: \(n_{HCl}=2n_{H2}=1,6\left(mol\right)\)
\(\rightarrow m_{HCl}=1,6.36,5=58,4\left(g\right)\)
\(\rightarrow m_{dd_{HCl}}=\frac{58,4}{7,3\%}=800\left(g\right)\)
Câu 5:
Gọi chung 2 kim loại là R hóa trị I
\(R+HCl\rightarrow RCl+\frac{1}{2}H_2\)
Ta có: \(n_{H2}=\frac{0,448}{22,4}=0,02\left(mol\right)\rightarrow n_{RCl}=2n_{H2}=0,04\left(mol\right)\)
\(\rightarrow m_{RCl}=0,04.\left(R+35,5\right)=2,58\rightarrow R=29\)
Vì 2 kim loại liên tiếp nhau \(\rightarrow\) 2 kim loại là Na x mol và K y mol
\(\rightarrow x+y=n_{RCl}=0,04\left(mol\right)\)
\(m_{hh}=m_R=23x+39y=0,04.29=1,16\left(g\right)\)
Giải được: \(\rightarrow\left\{{}\begin{matrix}x=0,025\\y=0,015\end{matrix}\right.\)
\(\rightarrow m_{Na}=0,575\left(g\right)\)
\(\rightarrow\%m_{Na}=\frac{0,575}{1,16}=49,57\%\rightarrow\%m_K=50,43\%\)
Câu 6:
Khối lượng mỗi phần là 35/2=17,5g
Gọi số mol Fe, Cu, Al là a, b, c
Ta có \(56a+64b=27c=17,5\)
Phần 1: \(n_{H2}=\frac{6,72}{22,4}=0,3\left(mol\right)\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(\Rightarrow a=1,5b=n_{H2}=0,3\)
Phần 2: \(n_{Cl2}=\frac{10,64}{22,4}=0,475\left(mol\right)\)
\(2Fe+3Cl_2\rightarrow2FeCl_3\)
\(Cu+Cl_2\rightarrow CuCl_2\)
\(2Al+3Cl_2\rightarrow2AlCl_3\)
\(\Rightarrow1,5a+b+1,5c=n_{Cl2}=0,465\)
\(\rightarrow\left\{{}\begin{matrix}a=0,15\\b=0,1\\c=0,1\end{matrix}\right.\)
\(\rightarrow\%m_{Fe}=\frac{0,15.56}{17,5}=48\%\)
\(\rightarrow\%m_{Cu}=\frac{0,1.64}{17,5}=36,57\%\)
\(\rightarrow\%m_{Al}=100\%-48\%-36,57\%=15,43\%\)
Câu 1
2Al+6HCl--->2Alcl3+3H2
x-----------------------1,5x
Zn+2HCl---->Zncl2+H2
y---------------------------y
n H2=1/2=0,5(mol)
Theo bài ta có hpt
\(\left\{{}\begin{matrix}27x+65y=18,4\\1,5x+y=0,5\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=0,2\\y=0,2\end{matrix}\right.\)
%m Al=0,2.27/18,4.100%=29,35%
%m Zn=100%-29,35=70,65%
Câu 2.
Fe+2HCl---->FeCl2+H2
FeO+2HCl--->FeCl2+H2
n H2=2,24/22,4=0,1(mol)
m H2=0,2(g)
n Fe=n H2=0,2(mol)
m Fe=0,2.56=11,2(g)
n FeCl2(1)=2n H2=0,2(mol)
m FeCl2(1)=0,2.127=25,4(g)
m FeCl2(PT2)=38,1-25,4=12,7(g)
n FeCl2=12,7/127=0,1(mol)
n FeO=n FeCl2=0,1(mol)
m FeO=0,1.72=7,2(g)
3.
Zn+2HCl--->ZnCl2+H2
n H2=4,48/22,4=0,2(mol)
n Zn=n H2=0,2(mol)
m Zn=0,2.56=11,2(g)
%m Zn=11,2/20.100%=56%
%m Cu=100-56=34%
b) n HCl=2n H2=0,4(mol)
V H2=0,4/2=0,2(l)
4.
a) Fe+2HCl---.FeCl2+H2
x-----------------------------x(mol)
2Al+6HCl--->AlCl3+3H2
y------------------------------1,5y
n H2=17,92/22,4=0,89mol)
Theo bài ta có hpt
\(\left\{{}\begin{matrix}56x+27y=22\\x+1,5y=0,8\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=0,2\\y=0,4\end{matrix}\right.\)
%m Fe=0,2.56/22.100%=50,9%
%m Al=100-50,9=49,1%
b) n HCl=2n H2=1,6(mol)
m HCl=1,6.36,5=58,4(g)
m dd HCl=58,4.100/7,3=800(g)
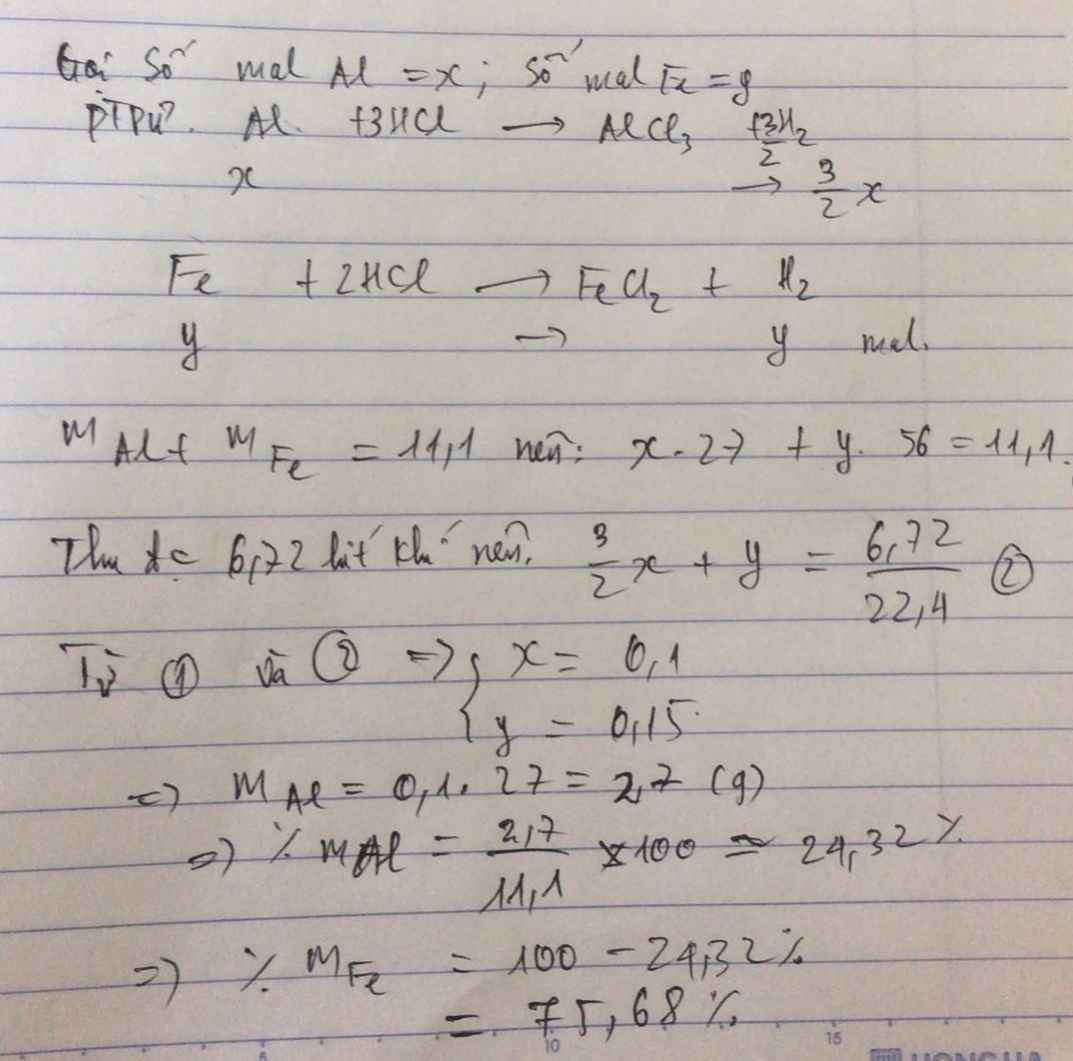


1)\(Mg+HCl\rightarrow MgCl_2+H_2\)
x____________________x
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
y ____________________1,5y
Giải hệ phương trình :
\(\left\{{}\begin{matrix}x+\frac{3}{2y}=0,5\\24x+27y=10,2\end{matrix}\right.\rightarrow x=y=0,2\)
\(\%_{Mg}=\frac{0,2.24}{10,2}.100\%=47,1\%\)
\(\rightarrow\%m_{Al}=100\%-47,1\%=52,9\%\)