Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, PT: \(Fe+H_2SO_4\rightarrow FeSO_4+H_2\)
b, Ta có: \(n_{Fe}=\dfrac{19,6}{56}=0,35\left(mol\right)\)
Theo PT: \(n_{H_2}=n_{Fe}=0,35\left(mol\right)\)
\(\Rightarrow V_{H_2}=0,35.22,4=7,84\left(l\right)\)
c, Ta có: \(n_{CuO}=\dfrac{12}{80}=0,15\left(mol\right)\)
PT: \(CuO+H_2\underrightarrow{t^o}Cu+H_2O\)
Xét tỉ lệ: \(\dfrac{0,15}{1}< \dfrac{0,35}{1}\), ta được H2 dư.
Theo PT: \(n_{H_2\left(pư\right)}=n_{CuO}=0,15\left(mol\right)\)
\(\Rightarrow n_{H_2\left(dư\right)}=0,35-0,15=0,2\left(mol\right)\)
\(\Rightarrow m_{H_2\left(dư\right)}=0,2.2=0,4\left(g\right)\)

a) Pt: \(Fe+2HCl\rightarrow FeCl_2+H_2\)
b) nFe = \(\dfrac{11,2}{56}=0,2mol\)
Theo pt: nH2 = nFe = 0,2 mol
=> VH2 = 0,2.22,4 = 4,48lit
c) Theo pt: nHCl = 2nFe = 0,4 mol
=> mHCl = 0,4.36,5 = 14,6 g
=> C% = \(\dfrac{14,6}{73}.100\%=20\%\)

a) Pt:
b) nFe = \(\dfrac{11,2}{56}=0,2mol\)
Theo pt: nH2 = nFe = 0,2 mol
=> VH2 = 0,2.22,4 = 4,48lit
c) Theo pt: nHCl = 2nFe = 0,4 mol
=> mHCl = 0,4.36,5 = 14,6 g
=> \(C\%=\dfrac{14,6}{73}.100\%=20\%\)

a,\(n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\)
PTHH: Fe + 2HCl → FeCl2 + H2
Mol: 0,2 0,4 0,2
b, \(V_{H_2}=0,2.22,4=4,48\left(l\right)\)
c, \(m_{HCl}=0,4.36,5=14,6\left(g\right)\)

\(n_{Fe}=\dfrac{11.2}{56}=0.2\left(mol\right)\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(0.2.......0.4....................0.2\)
\(V_{H_2}=0.2\cdot22.4=4.48\left(l\right)\)
\(m_{HCl}=0.4\cdot36.5=14.6\left(g\right)\)
PTHH : \(Fe+2HCl-->FeCl_2+H_2\uparrow\) (1)
\(n_{Fe}=\dfrac{m}{M}=\dfrac{11.2}{56}=0.2\left(mol\right)\)
Từ (1) => \(n_{Fe}=n_{H_2}=0.2\left(mol\right)\)
=> \(V_{H2\left(đktc\right)}=n.22,4=0,2.22,4=4,48\left(l\right)\)
Từ (1) => \(2n_{Fe}=n_{HCl}=0.4\left(mol\right)\)
=> \(m_{HCl}=n.M=0,4.\left(1+35.5\right)=14.6\left(g\right)\)

nFe = 16,8/56 = 0,3 (mol)
PTHH: Fe + 2HCl -> FeCl2 + H2
Mol: 0,3 ---> 0,6 ---> 0,3 ---> 0,3
VH2 = 0,3 . 24,79 = 7,437 (l)
mHCl = 0,6 . 36,5 = 21,9 (g)
PTHH: CuO + H2 -> (t°) Cu + H2O
Mol: 0,3 <--- 0,3 ---> 0,3
mCu = 0,3 . 64 = 19,2 (g)
mFe = 16,8: 56 =0,3(mol)
pthh : Fe + 2HCl --> FeCl2 + H2 (1)
0,3 ->0,6-----------------> 0,3 (mol)
=> VH2 (đkc) = 0,3 . 24,79 ( l)
=> mHCl = 0,6 . 35,5 = 21,9 (g)
pthh : CuO + H2 -t--> Cu+ H2O
0,3<-----0,3 (mol)
=>mCu = 0,3 . 64 = 19,2 (g)

\(a) Fe + 2HCl \to FeCl_2 + H_2\\ b) n_{FeCl_2} = n_{Fe} =\dfrac{11,2}{56} = 0,2(mol)\\ m_{FeCl_2} = 0,2.127 = 25,4(gam)\\ c) n_{H_2} = n_{Fe} = 0,2(mol)\Rightarrow V_{H_2} = 0,2.22,4 = 4,48(lít)\\ d) n_{HCl} = 2n_{Fe} = 0,4(mol)\\ C\%_{HCl} = \dfrac{0,4.36,5}{300}.100\% = 4,867\%\)

\(nAl=\dfrac{13,5}{27}=0,5\left(mol\right)\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
2 6 2 3 (mol)
0,5 1,5 0,5 0,75 (mol)
a. \(VH_2=0,75.22,4=16,8\left(l\right)\)
b.
\(FeO+H_2\rightarrow Fe+H_2O\)
1 1 1 1 (mol)
0,75 0,75 0,75 0,75
\(m_{Fe}=0,75.42\left(g\right)\)
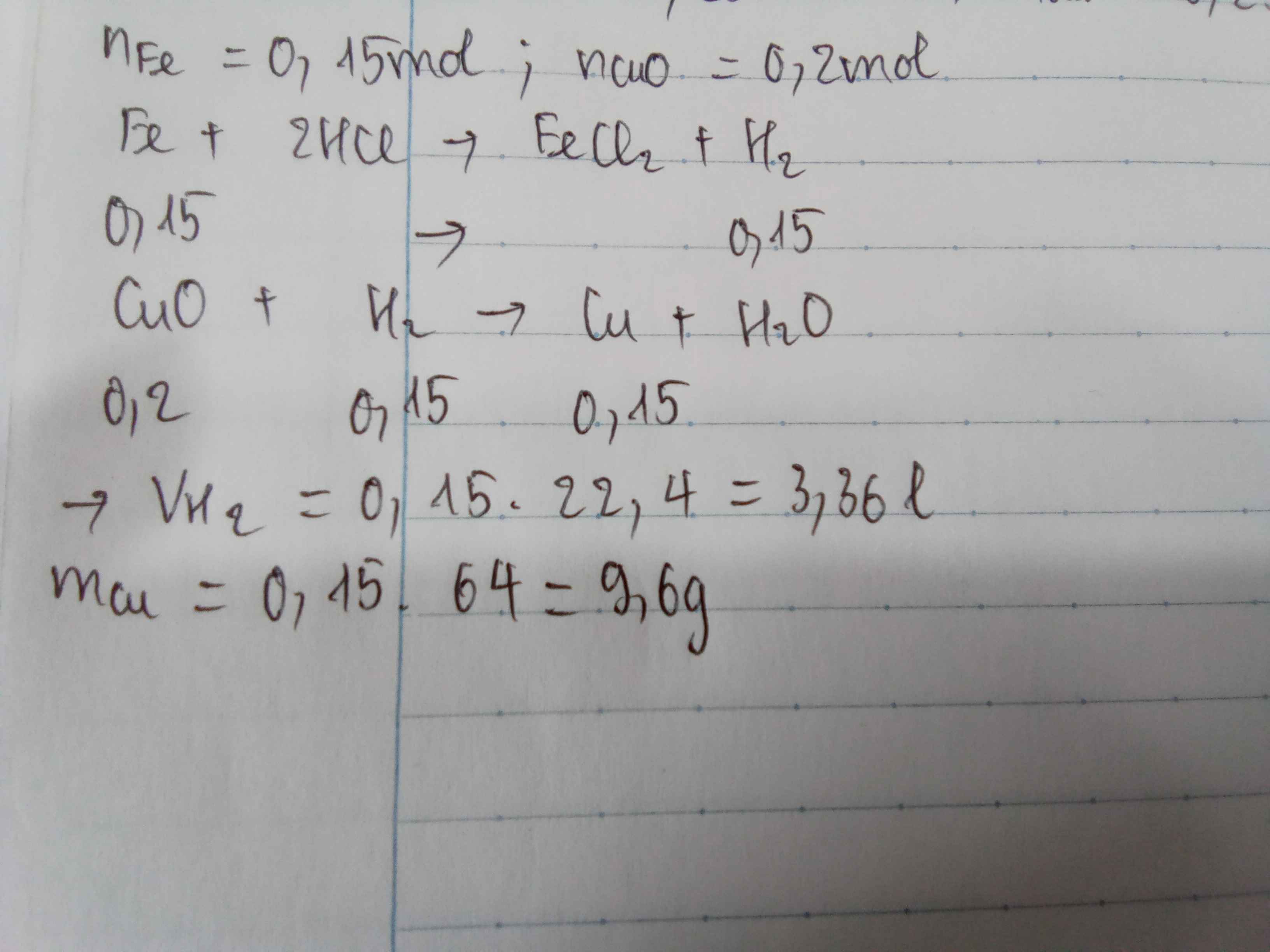

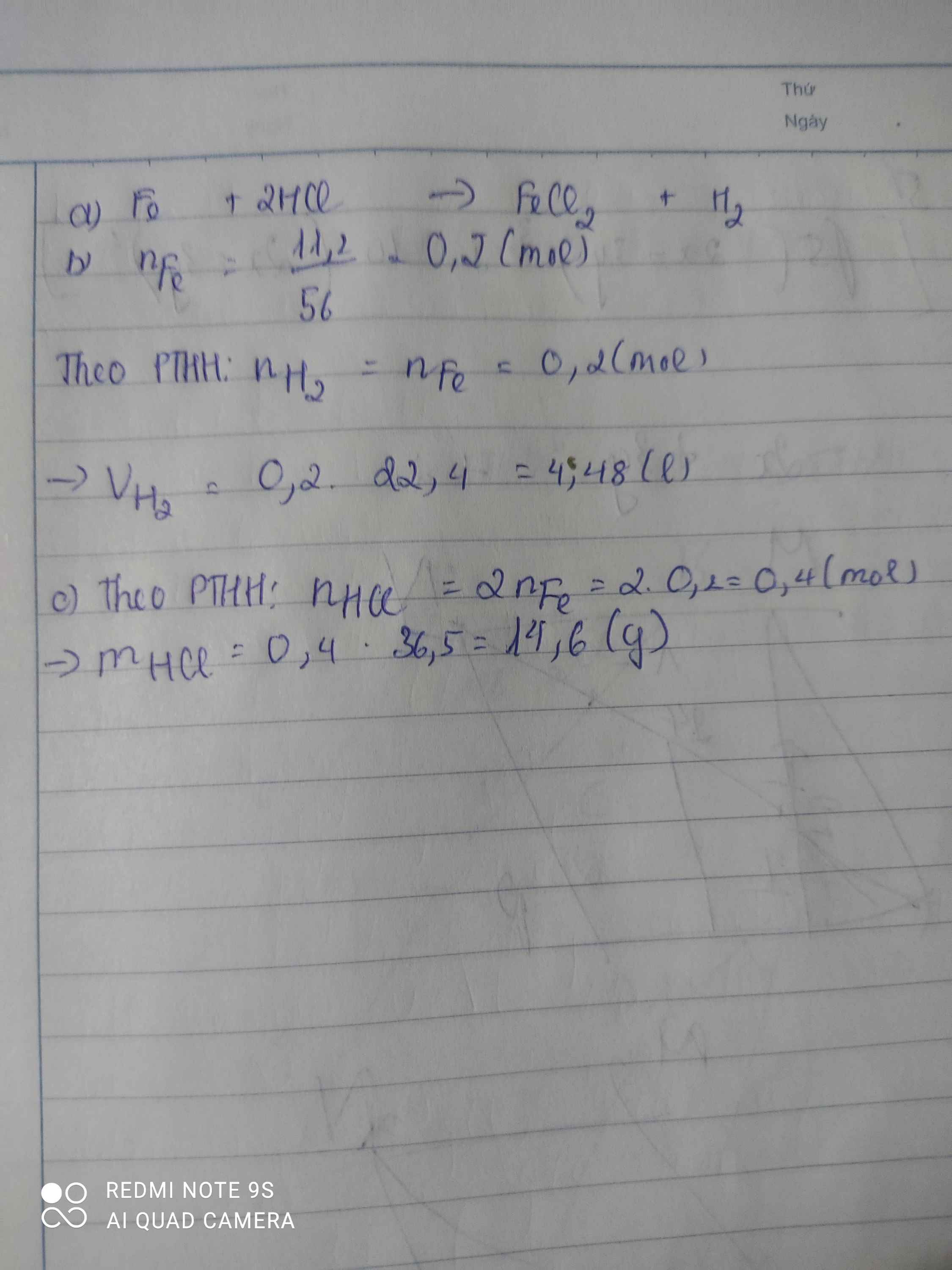
a) \(Fe+H2SO4-->FeSO4+H2\)
b)\(nFe=\frac{11,2}{56}=0,2\left(mol\right)\)
\(n_{H2}=n_{Fe}=0,2\left(mol\right)\)
\(VH2=0,2.22,4=4,48\left(l\right)\)
c)\(n_{FeSO4}=n_{Fe}=0,2\left(mol\right)\)
\(m_{FeSO4}=0,2.152=30,4\left(g\right)\)
d)\(CuO+H2-->Cu+H2O\)
\(n_{Cu}=n_{H2}=0,2\left(mol\right)\)
\(m_{Cu}=0,2.64=12,8\left(g\right)\)