Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a.b.c.\(n_{Al}=\dfrac{2,7}{27}=0,1mol\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
0,1 0,1 0,15 ( mol )
\(V_{H_2}=0,15.22,4=3,36l\)
\(m_{AlCl_3}=0,1.133,5=13,35g\)
d.\(Fe_2O_3+3H_2\rightarrow\left(t^o\right)2Fe+3H_2O\)
0,15 0,1 ( mol )
\(m_{Fe}=0,1.56=5,6g\)

\(n_{Fe}=\dfrac{16.8}{56}=0.3\left(mol\right)\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(0.3....................0.3.........0.3\)
\(m_{FeCl_2}=0.3\cdot127=38.1\left(g\right)\)
\(V_{H_2}=0.6\cdot22.4=6.72\left(l\right)\)
\(CuO+H_2\underrightarrow{t^0}Cu+H_2O\)
\(.......0.3...0.3\)
\(m_{Cu}=0.3\cdot64=19.2\left(g\right)\)
PT: \(Fe+2HCl\rightarrow FeCl_2+H_2\)
a, Ta có: \(n_{Fe}=\dfrac{16,8}{56}=0,3\left(mol\right)\)
Theo PT: \(n_{FeCl_2}=n_{Fe}=0,3\left(mol\right)\)
\(\Rightarrow m_{FeCl_2}=0,3.127=38,1\left(g\right)\)
b, Theo PT: \(n_{H_2}=n_{Fe}=0,3\left(mol\right)\)
\(\Rightarrow V_{H_2}=0,3.22,4=6,72\left(l\right)\)
c, PT: \(CuO+H_2\underrightarrow{t^o}Cu+H_2O\)
Theo PT: \(n_{Cu}=n_{H_2}=0,3\left(mol\right)\)
\(\Rightarrow m_{Cu}=0,3.64=19,2\left(g\right)\)
Bạn tham khảo nhé!

a.b.\(n_{Mg}=\dfrac{3,6}{24}=0,15mol\)
\(Mg+2HCl\rightarrow MgCl_2+H_2\)
0,15 0,3 0,15 ( mol )
\(V_{H_2}=0,15.22,4=3,36l\)
\(m_{HCl}=0,3.36,5=10,95g\)
c.\(n_{H_2}=0,15.60\%=0,09mol\)
\(Ag_2O+H_2\rightarrow\left(t^o\right)2Ag+H_2O\)
0,09 0,18 ( mol )
\(m_{Ag}=0,18.108=19,44g\)
\(a.n_{Mg}=\dfrac{3,6}{24}=0,15\left(mol\right)\\ Mg+2HCl\rightarrow MgCl_2+H_2\\ TheoPT:n_{H_2}=n_{Mg}=0,15\left(mol\right)\\ \Rightarrow V_{H_2}=0,15.22,4=3,36\left(l\right)\\ b.TheoPT:n_{HCl}=2n_{Mg}=0,3\left(mol\right)\\ \Rightarrow m_{Mg}=0,3.36,5=10,95\left(g\right)\\ c.n_{H_2\left(pứ\right)}=0,15.60\%=0,054\left(g\right)\\ H_2+Ag_2O-^{t^o}\rightarrow2Ag+H_2O\\ n_{Ag}=2n_{H_2}=0,108\left(mol\right)\\ \Rightarrow m_{Ag}=0,108.108=11,664\left(g\right)\)

a. \(PTHH:Mg+2HCl\rightarrow MgCl_2+H_2\uparrow\)
\(n_{Mg}=\dfrac{m_{Mg}}{M_{Mg}}=\dfrac{12}{24}=0,5\left(mol\right)\)
- Mol theo PTHH : \(1:2:1:1\)
- Mol theo phản ứng : \(0,5\rightarrow1\rightarrow0,5\rightarrow0,5\)
\(\Rightarrow n_{H_2}=0,5\left(mol\right)\)
\(\Rightarrow V_{H_2}=n_{H_2}.22,4=0,5.22,4=11,2\left(l\right)\)
b. Từ a. \(\Rightarrow n_{HCl}=1\left(mol\right)\)
\(\Rightarrow m_{HCl}=n_{HCl}.M_{HCl}=1.\left(1+35,5\right)=36,5\left(g\right)\)
c. \(n_{O_2}=\dfrac{V_{O_2}}{22,4}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
\(PTHH:2H_2+O_2\underrightarrow{t^o}2H_2O\)
- Mol theo PTHH : \(2:1:2\)
- Mol theo phản ứng : \(0,6\leftarrow0,3\rightarrow0,6\)
\(\Rightarrow n_{H_2O}=0,6\left(mol\right)\)
\(\Rightarrow m_{H_2O}=n_{H_2O}.M_{H_2O}=0,6.\left(2+16\right)=10,8\left(g\right)\)

a) \(n_{Mg}=\dfrac{4,8}{24}=0,2\left(mol\right)\)
PTHH: Mg + 2HCl --> MgCl2 + H2
0,2--------------------->0,2
=> VH2 = 0,2.22,4 = 4,48 (l)
b) \(n_{CuO}=\dfrac{24}{80}=0,3\left(mol\right)\)
PTHH: CuO + H2 --to--> Cu + H2O
Xét tỉ lệ: \(\dfrac{0,3}{1}>\dfrac{0,2}{1}\) => H2 hết, CuO dư
PTHH: CuO + H2 --to--> Cu + H2O
0,2<--0,2-------->0,2
=> mrắn sau pư = 24 - 0,2.80 + 0,2.64 = 20,8 (g)
c)
PTHH: RO + H2 --to--> R + H2O
0,2------>0,2
=> \(M_R=\dfrac{12,8}{0,2}=64\left(g/mol\right)\)
=> R là Cu
+) \(N_{Mg}\) = \(\dfrac{m}{M}\) = \(\dfrac{4,8}{24}\) = 0,2 mol
a) Mg + HCl -> \(MgCl_2\) + \(H_2\)
0,2 -> 0,2 (mol)
b) +) \(N_{CuO}\text{ }\)= \(\dfrac{m}{M}\) = \(\dfrac{24}{80}\) = 0,3 mol
+) \(H_2\) + CuO -> Cu + \(H_2O\)
+) Ta có: \(\dfrac{N_{H_2}}{1}\)= \(\dfrac{0,2}{1}\) < \(\dfrac{N_{CuO}}{1}\)= \(\dfrac{0,3}{1}\)
=> \(H_2\) hết. Tính toán theo \(N_{H_2}\)
+)\(H_2\) + CuO -> Cu + \(H_2O\)
Ban đầu: 0,2 0,3 0 0 }
P/ứng: 0,2 -> 0,2 -> 0,2 -> 0,2 } mol
Sau p/ư: 0 0,1 0,2 0,2 }
=> \(m_{Cu}\) = 12,8 gam .Thu được 2,8 gam Cu

Fe + 2HCl -> FeCl2 + H2
nFe = 5,6/56 = 0,1 mol
=>nH2 = 0,1 mol
=> VH2= 0,1*22,4= 2,24 lít
\(n_{Fe}=\dfrac{5,6}{56}=0,1\left(mol\right)\)
PTHH: Fe + 2HCl ---> FeCl2 + H2
0,1-->0,2------------------>0,1
=> \(\left\{{}\begin{matrix}m_{ddHCl}=\dfrac{0,2.36,5}{15\%}=\dfrac{146}{3}\left(g\right)\\V_{H_2}=0,1.22,4=4,48\left(l\right)\end{matrix}\right.\)

a.b.
\(n_{Fe}=\dfrac{2,8}{56}=0,05mol\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
0,05 0,1 0,05 ( mol )
\(V_{H_2}=0,05.22,4=1,12l\)
\(m_{HCl}=0,1.36,5=3,65g\)
c.
\(CuO+H_2\rightarrow\left(t^o\right)Cu+H_2O\)
0,05 0,05 ( mol )
\(m_{Cu}=0,05.64=3,2g\)
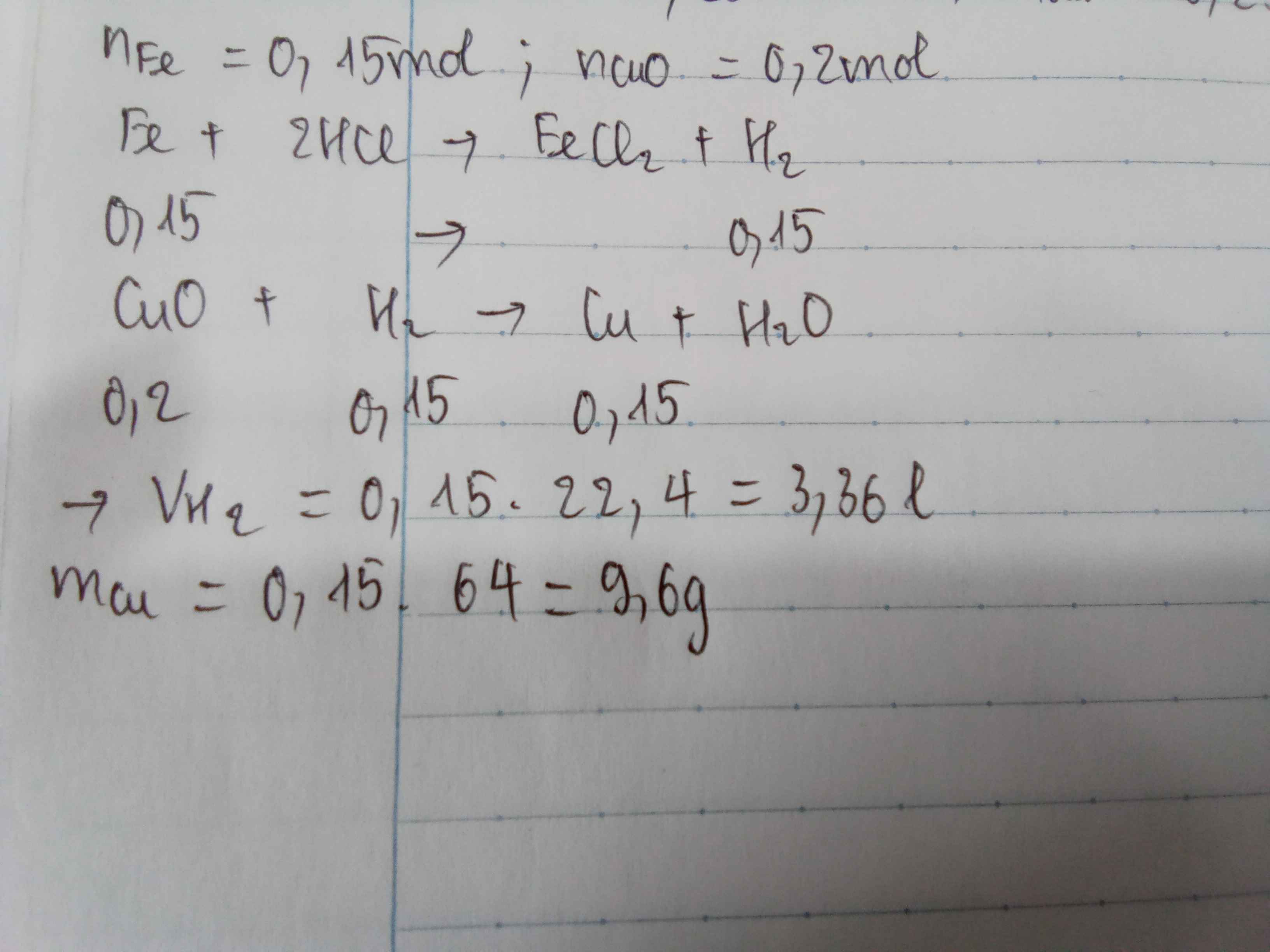

nFe = 16,8/56 = 0,3 (mol)
PTHH: Fe + 2HCl -> FeCl2 + H2
Mol: 0,3 ---> 0,6 ---> 0,3 ---> 0,3
VH2 = 0,3 . 24,79 = 7,437 (l)
mHCl = 0,6 . 36,5 = 21,9 (g)
PTHH: CuO + H2 -> (t°) Cu + H2O
Mol: 0,3 <--- 0,3 ---> 0,3
mCu = 0,3 . 64 = 19,2 (g)
mFe = 16,8: 56 =0,3(mol)
pthh : Fe + 2HCl --> FeCl2 + H2 (1)
0,3 ->0,6-----------------> 0,3 (mol)
=> VH2 (đkc) = 0,3 . 24,79 ( l)
=> mHCl = 0,6 . 35,5 = 21,9 (g)
pthh : CuO + H2 -t--> Cu+ H2O
0,3<-----0,3 (mol)
=>mCu = 0,3 . 64 = 19,2 (g)