Cho a gam Zn vào 200g đ HCl thoát ra 3,36l khí H2(dktc) A) tìm m gam Zn B)Tìm m zncl2 C) Tính C% đ hcl
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


a. \(n_{Zn}=\dfrac{19.5}{65}=0,3\left(mol\right)\)
\(n_{HCl}=\dfrac{14.6}{36.5}=0,4\left(mol\right)\)
PTHH : Zn + 2HCl -> ZnCl2 + H2
0,4 0,2 0,2
Ta thấy : \(\dfrac{0.3}{1}>\dfrac{0.4}{2}\) => Zn dư , HCl đủ
b. \(V_{H_2}=0,2.22,4=4,48\left(l\right)\)
c. \(m_{ZnCl_2}=0,2.136=27,2\left(g\right)\)
a) Zn + 2HCl --> ZnCl2 + H2 ↑ (1)
0,3 -->0,15 -->0,15 (mol)
nZn= 19,5/65 = 0,3 mol
nHCl= 14,5/37,5 = 0,3 mol
Ta có : nZn bài ra / nZn phương trình=0,3/1=0,3 (mol)
nHCl bài ra / nHCl phương trình=0,3/2=0,15 (mol)
=> HCl đủ,Zn dư
b) Theo PT(1) => nH2=0,15(mol)
=>VH2=0,15 x 22,4 = 3,36(l)
c) Theo PT(1) => nZnCl2=0,15(mol)
=>mZnCl2=0,15 x 136 = 20,4(g)

\(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\\
pthh:Zn+2HCl\rightarrow ZnCl_2+H_2\)
0,1 0,2 0,1 0,1
\(m_{HCl}=0,2.36,5=7,3\left(g\right)\\
V_{H_2}=0,1.22,4=2,24l\\
m_{\text{dd}}=6,5+200-\left(0,1.2\right)=206,3g\)
bài 2 :
\(n_{Mg}=\dfrac{4,8}{24}=0,2\left(mol\right)\\
pthh:Mg+2HCl\rightarrow MgCl_2+H_2\)
0,2 0,4 0,2 0,2
\(m_{HCl}=0,4.36,5=14,6g\\
V_{H_2}=0,2.22,4=4,48l\\
m\text{dd}=4,8+200-0,4=204,4g\\
C\%=\dfrac{0,2.136}{204,4}.100\%=13,3\%\)

\(n_{Zn}=\dfrac{6,5}{65}=0,1mol\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
0,1 0,2 0,1 0,1
\(V_{H_2}=0,1\cdot22,4=2,24l\)

`Zn + 2HCl -> ZnCl_2 + H_2`
`0,1` `0,2` `0,1` `(mol)`
`n_[Zn]=[6,5]/65=0,1(mol)`
`a)V_[H_2]=0,1.22,4=2,24(l)`
`b)C%_[HCl]=[0,2.36,5]/200 . 100 =3,65%`
`Zn + HCl -> ZnCl_2 + H_2` `\uparrow`
`n_(Zn) = (6,5)/65 = 0,1 mol`.
`n_(H_2) = 0,1 mol`.
`V(H_2) = 0,1 xx 22,4 = 2,24l`.
`C%(HCl) = (0,2.36,5)/200 xx 100 = 36,5%`.

\(m_{HCl}=219.10\%=21,9\left(g\right)\Rightarrow n_{HCl}=\dfrac{21,9}{36,5}=0,6\left(mol\right)\)
PT: \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
Theo PT: \(n_{ZnCl_2}=n_{H_2}=\dfrac{1}{2}n_{HCl}=0,3\left(mol\right)\)
a, \(V_{H_2}=0,3.24,79=7,437\left(l\right)\)
b, \(m_{ZnCl_2}=0,3.136=40,8\left(g\right)\)

\(n_{H_2}=\dfrac{4.48}{22.4}=0.2\left(mol\right)\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(0.2.......0.4.......................0.2\)
\(m_{Zn}=0.2\cdot65=13\left(g\right)\)
\(C\%_{HCl}=\dfrac{0.4\cdot36.5}{200}\cdot100\%=7.3\%\)
\(n_{CuO}=\dfrac{24}{80}=0.3\left(mol\right)\)
\(CuO+H_2\underrightarrow{^{t^0}}Cu+H_2O\)
\(1..........1\)
\(0.3.........0.2\)
\(LTL:\dfrac{0.3}{1}>\dfrac{0.2}{1}\Rightarrow CuOdư\)
\(m_{CuO\left(dư\right)}=\left(0.3-0.2\right)\cdot64=6.4\left(g\right)\)

Zn + 2HCl -> ZnCl2 + H2 (1)
nZn=0,1(mol)
Từ 1:
nZnCl2=nH2=nZn=0,1(mol)
mZnCl2=136.0,1=13,6(g)
VH2=0,1.22,4=2,24(lít)
CuO +H2 -> Cu + H2O (2)
Từ 2:
nO=nH2=0,1(mol)
mO=16.0,1=1,6(g)
mchất rắn còn lại=10-1,6=8,4(g)
Chúc Bạn Học Tốt

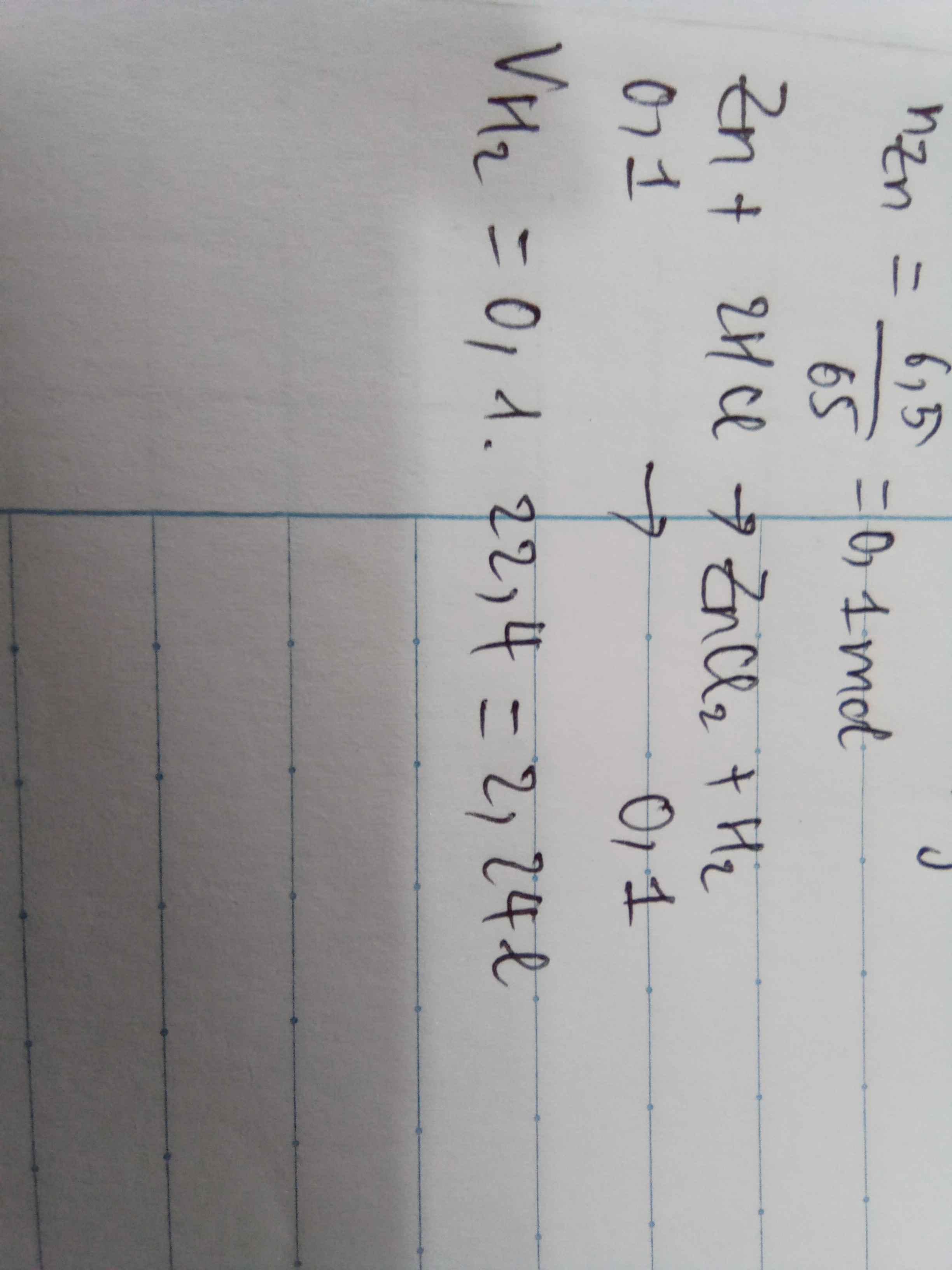
\(n_{H2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
Pt : \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
0,15<---0,3<-----0,15<-----0,15
a) \(m_{Zn}=0,15.65=9,75\left(g\right)\)
b) \(m_{ZnCl2}=0,15.136=20,4\)
c) \(C\%_{ddHCl}=\dfrac{0,3.36,5}{200}.100\%=5,475\%\)
\(n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
PT: \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
a, \(n_{Zn}=n_{H_2}=0,15\left(mol\right)\Rightarrow m_{Zn}=0,15.65=9,75\left(g\right)\)
b, \(n_{ZnCl_2}=n_{H_2}=0,15\left(mol\right)\Rightarrow m_{ZnCl_2}=0,15.136=20,4\left(g\right)\)
c, \(n_{HCl}=2n_{H_2}=0,3\left(mol\right)\Rightarrow C\%_{HCl}=\dfrac{0,3.36,5}{200}.100\%=5,475\%\)