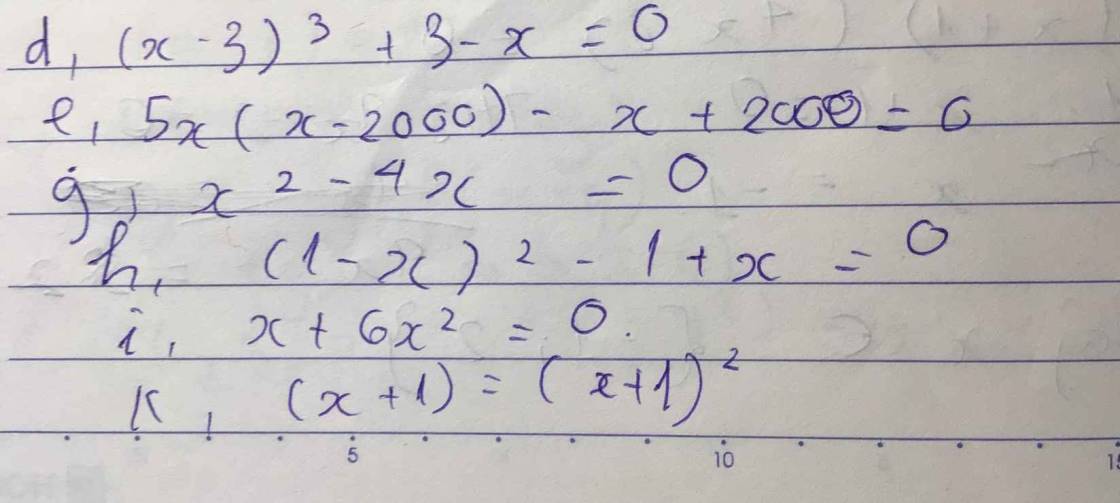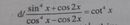
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Đặt tích 3 số tự nhiên liên tiếp là a * (a + 1) * (a + 2)
+Nếu a = 2k thì:
a * (a + 1) * (a + 2) chia hết cho 2
+ Nếu a = 2k +1 thì:
a+1=2k+1+1=2k+2 chia hết cho 2
Suy ra a * (a + 1) * (a + 2) chia hết cho 2
+ Nếu a = 3k thì
a * (a + 1) * (a + 2) chia hết cho 3
+ Nếu a = 3k +1 thì
a+2=3k+1+2=3k+3 chia hết cho 3
Suy ra a * (a + 1) * (a + 2) chia hết cho 3
+ Nếu a = 3k+2 thì:
a+1=3k+2+1=3k+3 chia hết cho 3
Suy ra a * (a + 1) * (a + 2) chia hết cho 3
Vì 2 và 3 nguyên tố cùng nhau nên a * (a + 1) * (a + 2) chia hết cho 2.3=6 (đpcm)

1
\(n_{Fe}=\dfrac{5,6}{56}=0,1\left(mol\right)\\
pthh:Fe+H_2SO_4\rightarrow FeSO_4+H_2\uparrow\)
0,1 0,1 0,1 0,1
\(V_{H_2}=0,1.22,4=2,24\left(l\right)\\
m_{FeSO_4}=127.0,1=12,7\left(g\right)\)
\(m_{\text{dd}}=5,6+500-\left(0,1.2\right)=505,4\left(g\right)\\
C\%_{FeSO_4}=\dfrac{12,7}{505,4}.100\%=2,513\%\)
2
\(n_{Mg}=\dfrac{4,8}{24}=0,2\left(mol\right)\\
pthh:Mg+H_2SO_4\rightarrow MgSO_4+H_2\uparrow\)
0,2 0,2 0,2 0,2
\(V_{H_2}=0,2.22,4=4,48\left(l\right)\\
m_{MgSO_4}=120.0,2=24\left(g\right)\\
V_{\text{dd}H_2SO_4}=\dfrac{0,2}{1}=0,2M\)

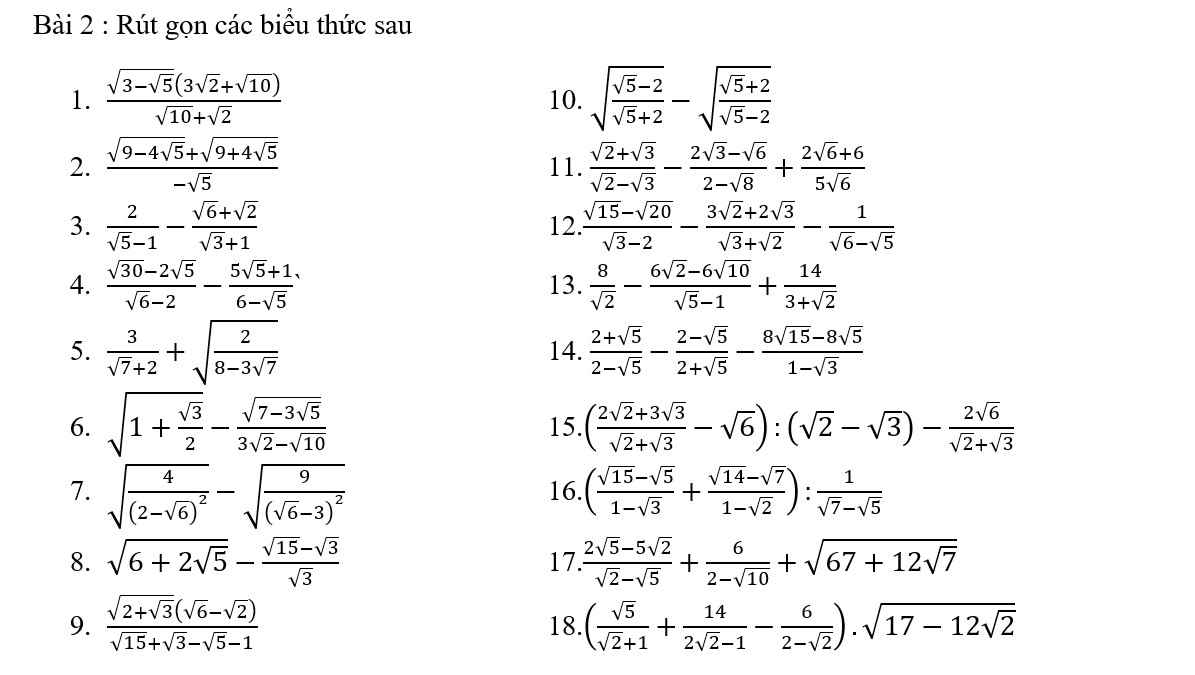
8: Ta có: \(\sqrt{6+2\sqrt{5}}-\dfrac{\sqrt{15}-\sqrt{3}}{\sqrt{3}}\)
\(=\sqrt{5}+1-\sqrt{5}+1\)
=2

Câu 10:
\(n_{Na_2O}=\dfrac{6,2}{62}=0,1\left(mol\right)\\ Na_2O+H_2O\rightarrow2NaOH\\ n_{NaOH}=0,1.2=0,2\left(mol\right)\\ a,C_{M\text{dd}NaOH}=\dfrac{0,2}{0,4}=0,5\left(M\right)\\ b,2NaOH+MgCl_2\rightarrow Mg\left(OH\right)_2+2NaCl\\ n_{MgCl_2}=2.0,2=0,4\left(mol\right)\\ V\text{ì}:\dfrac{0,2}{2}< \dfrac{0,4}{1}\Rightarrow MgCl_2d\text{ư}\\ n_{Mg\left(OH\right)_2}=\dfrac{0,2}{2}=0,1\left(mol\right)\\ m_{Mg\left(OH\right)_2}=m_{\downarrow}=0,1.58=5,8\left(g\right)\)
Câu 7:
\(n_{CO_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\\ n_{Ca\left(OH\right)_2}=0,25.2=0,5\left(mol\right)\\ V\text{ì}:1>\dfrac{n_{CO_2}}{n_{Ca\left(OH\right)_2}}=\dfrac{0,3}{0,5}=0,6\Rightarrow Ca\left(OH\right)_2d\text{ư}\\ Ca\left(OH\right)_2+CO_2\rightarrow CaCO_3\downarrow+H_2O\\ n_{CaCO_3}=n_{CO_2}=0,3\left(mol\right)\\ m_{CaCO_3}=100.0,3=30\left(g\right)\)

\(1,\left\{{}\begin{matrix}3x-y=5\\5x+2y=23\end{matrix}\right.\\ \Leftrightarrow\left\{{}\begin{matrix}y=3x-5\\5x+2\left(3x-5\right)=23\end{matrix}\right.\\ \Leftrightarrow\left\{{}\begin{matrix}y=3x-5\\5x+6x-10=23\end{matrix}\right.\\ \Leftrightarrow\left\{{}\begin{matrix}y=3x-5\\11x=33\end{matrix}\right.\\ \Leftrightarrow\left\{{}\begin{matrix}y=3.3-5\\x=3\end{matrix}\right.\\ \Leftrightarrow\left\{{}\begin{matrix}y=4\\x=3\end{matrix}\right.\)
\(2,\left\{{}\begin{matrix}5x-4y=3\\2x+y=4\end{matrix}\right.\\ \Leftrightarrow\left\{{}\begin{matrix}5x-4\left(4-2x\right)=3\\y=4-2x\end{matrix}\right.\\ \Leftrightarrow\left\{{}\begin{matrix}5x-16+8x=3\\y=4-2x\end{matrix}\right.\\ \Leftrightarrow\left\{{}\begin{matrix}13x=19\\y=4-2x\end{matrix}\right.\\ \Leftrightarrow\left\{{}\begin{matrix}x=\dfrac{19}{13}\\y=4-2.\dfrac{19}{13}\end{matrix}\right.\\ \Leftrightarrow\left\{{}\begin{matrix}x=\dfrac{19}{13}\\y=\dfrac{14}{13}\end{matrix}\right.\)

\(a,m=3\Leftrightarrow y=2x+2\\ A\left(a;-4\right)\in\left(d\right)\Leftrightarrow2a+2=-4\Leftrightarrow a=-3\)
\(b,\) PT giao Ox của (d) là \(2x+m-1=0\Leftrightarrow x=\dfrac{1-m}{2}\Leftrightarrow M\left(\dfrac{1-m}{2};0\right)\Leftrightarrow OM=\dfrac{\left|1-m\right|}{2}\)
PT giao Oy của (d) là \(x=0\Leftrightarrow y=m-1\Leftrightarrow N\left(0;m-1\right)\Leftrightarrow ON=\left|m-1\right|\)
Để \(S_{OMN}=1\Leftrightarrow\dfrac{1}{2}OM\cdot ON=1\Leftrightarrow OM\cdot ON=2\)
\(\Leftrightarrow\dfrac{\left|\left(1-m\right)\left(m-1\right)\right|}{2}=2\\ \Leftrightarrow\left|-\left(m-1\right)^2\right|=2\\ \Leftrightarrow\left(m-1\right)^2=2\\ \Leftrightarrow\left[{}\begin{matrix}m=1+\sqrt{2}\\m=1-\sqrt{2}\end{matrix}\right.\)




 giúp em 2 câu này vs ạ ngày mai nộp rồi nhưng vẫn ko bt cách làm.Em cảm ơn trc ạ
giúp em 2 câu này vs ạ ngày mai nộp rồi nhưng vẫn ko bt cách làm.Em cảm ơn trc ạ