Tính thành phần % về khối lượng các nguyên tố trong hợp chất: Fe2O3
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(CaCO_3\\ \%m_{Ca}=\dfrac{40}{40+12+3.16}.100=40\%\\ \%m_C=\dfrac{12}{40+12+16.3}.100=12\%\\ \Rightarrow\%m_O=100\%-\left(40\%+12\%\right)=48\%\\ H_2SO_4\\ \%m_H=\dfrac{2.1}{2.1+32+4.16}.100\approx2,041\%\\ \%m_S=\dfrac{32}{2.1+32+4.16}.100\approx32,653\%\\ \%m_O=\dfrac{4.16}{2.1+32+4.16}.100\approx65,306\%\\ Fe_2O_3\\ \%m_{Fe}=\dfrac{56.2}{56.2+16.3}.100=70\%\\ \Rightarrow\%m_O=100\%-70\%=30\%\)
CaCO3
\(\%M_{\dfrac{Ca}{CaCO_3}}=\dfrac{40}{100}.100\%=40\%\)
\(\%M_{\dfrac{C}{CaCO_3}}=\dfrac{12}{100}.100\%=12\%\)
\(\%M_{\dfrac{O}{CaCO_3}}=100\%-\left(40\%+12\%\right)=48\%\)
H2SO4
\(\%M_{\dfrac{H_2}{H_2SO_4}}=\dfrac{2}{98}.100\%=2,04\%\)
\(\%M_{\dfrac{S}{H_2SO_4}}=\dfrac{32}{98}.100\%=32,65\%\)
\(\%M_{\dfrac{O}{H_2SO_4}}=100\%-\left(2,04\%+32,65\%\right)=65,31\%\)
Fe2O3
\(\%M_{\dfrac{Fe}{Fe_2O_3}}=\dfrac{112}{160}.100\%=70\%\)
\(\%M_{\dfrac{O}{Fe_2O_3}}=100\%-70\%=30\%\)

`a,` \(K.L.P.T_{Fe_2O_3}=56.2+16.3=160< amu>.\)
\(\%Fe=\dfrac{56.2.100}{160}=70\%\)
\(\%O=100\%-70\%=30\%\)
`b,`\(K.L.P.T_{CaCO_3}=40+12+16.3=100< amu>.\)
\(\%Ca=\dfrac{40.100}{100}=40\%\)
\(\%C=\dfrac{12.100}{100}=12\%\)
\(\%O=100\%-40\%-12\%=48\%\)
`c,` \(K.L.P.T_{HCl}=1+35,5=36,5< amu>.\)
\(\%H=\dfrac{1.100}{36,5}\approx2,74\%\)
\(\%Cl=100\%-2,74\%=97,26\%\)
a: \(\%Fe=\dfrac{56\cdot2}{56\cdot2+16\cdot3}=70\%\)
=>%O=30%
b: \(\%Ca=\dfrac{40}{40+12+16\cdot3}=40\%\)
\(\%C=\dfrac{12}{100}=12\%\)
%O=100%-12%-40%=48%
c: %H=1/36,5=2,74%
=>%Cl=97,26%

\(M_{Fe_2O_3}=56\cdot2+16\cdot3=160\left(đvc\right)\)
\(\%m_{Fe}=\dfrac{112}{160}\cdot100\%=70\%\)
\(\%m_O=\dfrac{48}{160}\cdot100\%=30\%\)
\(\left\{{}\begin{matrix}\%Fe=\dfrac{56.2}{56.2+16.3}.100\%=70\%\\\%O=100\%-70\%=30\%\end{matrix}\right.\)

`@` `\text {MgO}`
\(\text{PTK = 24 + 16 = 40 < amu>}\)
\(\%\text{O}=\dfrac{16\cdot100}{40}=40\%\)
Vậy, khối lượng `%` của `\text {O}` trong `\text {MgO}` là `40%`
`@` `\text {Fe}_2 \text {O}_3`
\(\text{PTK = }56\cdot2+16\cdot3=160\text{ }< \text{amu}\text{ }>\)
\(\%\text{Fe}=\dfrac{56\cdot2\cdot100}{160}=70\%\)
Vậy, khối lượng `%` của `\text {Fe}` trong `\text {Fe}_2 \text {O}_3` là `70%`

- Hợp chất SO3
%S = MS : MSO3 .100% = 32 : 80 .100% = 40%
%O = 100% - 40% = 60%
- Hợp chất Fe2O3
%Fe = 2MFe : MFe2O3 . 100% = 2.56 : 160. 100% = 70%
%O = 100% - 70% = 30%
- Hợp chất CO2
%C = MC : MCO2 . 100% = 12 : 44 . 100% = 27,3 %
%O = 100% - 27,3% = 72,7%
\(\begin{array}{l} *SO_3:\\ \%S=\dfrac{32}{32+16\times 3}\times 100\%=40\%\\ \%O=\dfrac{16\times 3}{32+16\times 3}\times 100\%=60\%\\ *Fe_2O_3:\\ \%Fe=\dfrac{56\times 2}{56\times 2+16\times 3}\times 100\%=70\%\\ \%O=\dfrac{16\times 3}{56\times 2+16\times 3}\times 100\%=30\%\\ *CO_2:\\ \%C=\dfrac{12}{12+16\times 2}\times 100\%=27,27\%\\ \%O=\dfrac{16\times 2}{12+16\times 2}\times 100\%=72,73\%\end{array}\)

\(\%Fe=\dfrac{56}{56.2+16.3}.100\%=35\%\\ \%O=\dfrac{16}{56.2+16.3}.100\%=10\%\)
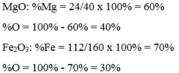
\(\%Fe=\dfrac{m_{Fe}}{M_{Fe_2O_3}}=\dfrac{112}{160}=70\%\\ \%O=100\%-\%Fe=100\%-70\%=30\%\)
cảm ơn