Viết Pthh chuyển đổi dãy số sau Fe->Fe2(SO4)3->Fe(OH)3->Fe2O3->Fe->Fecl2->Fe(NO3)2 Em xin cảm ơn ạ
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(a,2Fe+3Cl_2\xrightarrow{t^o}2FeCl_3\\ FeCl_3+3NaOH\to Fe(OH)_3\downarrow+3NaCl\\ 2Fe(OH)_3\xrightarrow{t^o}Fe_2O_3+3H_2O\\ Fe_2O_3+3CO\xrightarrow{t^o}2Fe+3CO_2\\ Fe+H_2SO_4\to FeSO_4+H_2\\ FeSO_4+BaS\to BaSO_4\downarrow+FeS\\ FeS+2HCl\to FeCl_2+H_2\\ FeCl_2+2AgNO_3\to Fe(NO_3)_2+2AgCl\downarrow\)
\(b,2Al_2O_3\xrightarrow[criolit]{đpnc}4Al+3O_2\\ 2Al+3H_2SO_4\to Al_2(SO_4)_3+3H_2\\ Al_2(SO_4)_3+6NaOH\to 2Al(OH)_3\downarrow+3Na_2SO_4\\ Al(OH)_3+3HNO_3\to Al(NO_3)_3+3H_2O\\ 2Al(NO_3)_3+3Mg\to 3Mg(NO_3)_2+2Al\\ Al+NaOH+H_2O\to NaAlO_2+\dfrac{3}{2}H_2\)

\(Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
\(2Fe+3Cl_2\underrightarrow{t^o}2FeCl_3\)
\(FeCl_3+3NaOH\rightarrow3NaCl+Fe\left(OH\right)_3\)
\(2Fe\left(OH\right)_3+3H_2SO_4\rightarrow Fe_2\left(SO_4\right)_3+6H_2O\)

a) \(Ba\rightarrow Ba\left(OH\right)_2\rightarrow BaCl_2\rightarrow Ba\left(NO_3\right)_2\rightarrow BaCO_3\)
\(Ba+2H_2O\rightarrow Ba\left(OH\right)_2+H_2\uparrow\)
\(Ba\left(OH\right)_2+2HCl\rightarrow BaCl_2+2H_2O\)
\(BaCl_2+2AgNO_3\rightarrow Ba\left(NO_3\right)_2+2AgCl\downarrow\)
\(Ba\left(NO_3\right)_2+K_2CO_3\rightarrow2KNO_3+BaCO_3\downarrow\)
b) \(Fe\rightarrow Fe_3O_4\rightarrow Fe_2O_3\rightarrow Fe_2\left(SO_4\right)_3\rightarrow FeCl_3\rightarrow Fe\left(OH\right)_3\rightarrow Fe\left(NO_3\right)_3\)
\(3Fe+2O_2\xrightarrow[]{t^o}Fe_3O_4\)
\(2Fe_3O_4+\dfrac{1}{2}O_2\xrightarrow[]{t^o}3Fe_2O_3\)
\(Fe_2O_3+3H_2SO_4\rightarrow Fe_2\left(SO_4\right)_3+3H_2O\)
\(Fe_2\left(SO_4\right)_3+3BaCl_2\rightarrow2FeCl_3+3BaSO_4\downarrow\)
\(FeCl_3+3KOH\rightarrow Fe\left(OH\right)_3\downarrow+3KCl\)
\(Fe\left(OH\right)_3+3HNO_3\rightarrow Fe\left(NO_3\right)_3+3H_2O\)

a/ Fe(NO3)3 –> Fe(OH)3 –> Fe2O3 –> FeCl3 –> Fe –> FeCl2 –> AgCl
Fe(NO3)3+3NaOH->3NaNO3+Fe(OH)3
2Fe(OH)3-to>Fe2O3+3H2O
Fe2O3+6HCl->2Fecl3+3H2O
2FeCl3+3Mg->3Mgcl2+2Fe
Fe+2HCl->Fecl2+H2
FeCl2+2AgNO3->2AgCl+Fe(NO3)2

\(2Fe\left(OH\right)_3-^{t^o}\rightarrow Fe_2O_3+3H_2O\\ Fe_2O_3+3H_2SO_4\rightarrow Fe_2\left(SO_4\right)_3+3H_2O\\ Fe_2\left(SO_4\right)_3+3BaCl_2\rightarrow2FeCl_3+3BaSO_4\\ FeCl_3+3NaOH\rightarrow Fe\left(OH\right)_3+3NaCl\)

\(FeCl_2+2NaOH\to Fe(OH)_2\downarrow+2NaCl\\ 4Fe(OH)_2+2H_2O+O_2\xrightarrow{t^o}4Fe(OH)_3\\ 2Fe(OH)_3\xrightarrow{t^o}Fe_2O_3+3H_2O\\ Fe_2O_3+3CO\xrightarrow{t^o}2Fe+3CO_2\)

\(2Fe + 3Cl_2 \xrightarrow{t^o} 2FeCl_3\\ FeCl_3 + 3KOH \to Fe(OH)_3 + 3KCl\\ Fe(OH)_3 + 3HNO_3 \to Fe(NO_3)_3 + 3H_2O\\ Fe(NO_3)_3 + 3KOH \to 3KNO_3 + Fe(OH)_3\\ 2Fe(OH)_3 \xrightarrow{t^o} Fe_2O_3 + 3H_2O\\ Fe_2O_3 + 3H_2SO_4 \to Fe_2(SO_4)_3 + 3H_2O\)
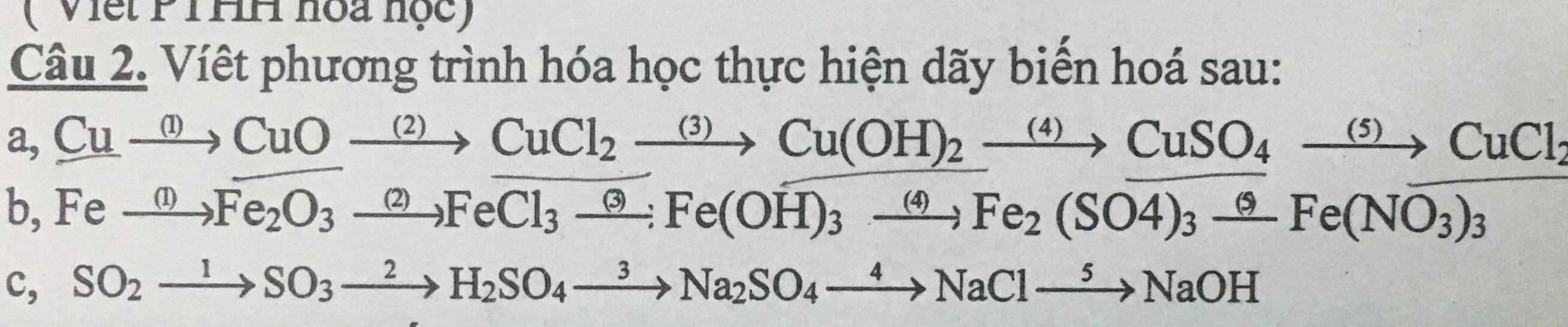
\(Fe\underrightarrow{1}Fe_2\left(SO_4\right)_3\underrightarrow{2}Fe\left(OH\right)_3\underrightarrow{3}Fe_2O_3\underrightarrow{4}Fe\underrightarrow{5}FeCl_2\underrightarrow{6}Fe\left(NO_3\right)_2\)
(1) \(2Fe+6H_2SO_{4đặc}\underrightarrow{t^o}Fe_2\left(SO_4\right)_3+3SO_2+6H_2O\)
(2) \(Fe_2\left(SO_4\right)_3+6NaOH\rightarrow2Fe\left(OH\right)_3+3Na_2SO_4\)
(3) \(2Fe\left(OH\right)_3\underrightarrow{t^o}Fe_2O_3+3H_2O\)
(4) \(Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
(5) \(Fe+2HCl\rightarrow FeCl_2+H_2\)
(6) \(FeCl_2+2AgNO_3\rightarrow Fe\left(NO_3\right)_2+2AgCl\)
Chúc bạn học tốt