fe + hno3 -> fe(no3)3 +NxOy +h2o
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.



a) 3M + 4nHNO3 → 3M(NO3)n + nNO + 2nH2O
b) 2M + 2nH2SO4 → M2(SO4)n + nSO2 + 2nH2O
c) 8M + 30HNO3 → 8M(NO3)3 + 3N2O + 15H2O
d) 8M + 10nHNO3 → 8M(NO3)n + nN2O + 5nH2O
e) (5x−2y)Fe + (15x−3y)HNO3 → (5x−2y)Fe(NO3)3 + 3NxOy + (15x−3y/2)H2O


a)\(3M+4nHNO_3-->3M\left(NO_3\right)_n+nNO+2nH_2O\)
b)
\(2M+2nH_2SO_4-->M_2\left(SO_4\right)_n+nSO_2+2nH_2O\)
c)
\(8M+30HNO_3-->8M\left(NO_3\right)_3+3N_2O+15H_2O\)
d)
\(8M+10nHNO_3-->8M\left(NO_3\right)_n+nN_2O+5nH_2O\)
e)\(\left(5x-2y\right)Fe+\left(15x-3y\right)HNO_3-->\left(5x-2y\right)Fe\left(NO_3\right)_3+3N_xO_y+\left(\dfrac{15x-3y}{2}\right)H_2O\)
f) \(3Fe_xO_y+\left(6x+2y\right)HNO_3-->3xFe\left(NO_3\right)_3+\left(2y-3x\right)NO+\left(3x+y\right)H_2O\)
g)\(Fe_xO_y+\left(6x-2y\right)HNO_3-->xFe\left(NO_3\right)_3+\left(3x-2y\right)NO_2+\left(3x-y\right)H_2O\) h)\(Fe_xO_y+2yHCl-->xFeCl_{\dfrac{2y}{x}}+yH_2O\)
i)\(2Fe_xO_y+2yH_2SO_4-->xFe_2\left(SO_4\right)_{\dfrac{2y}{x}}+2yH_2O\)

Al->Al+3 +3e________________ .(5x-2y)
xN+5 +(5x-2y)e\(\rightarrow\)xN+2y/x________.3
\(\Rightarrow\)(5x-2y)Al+(18x-3y)HNO3\(\rightarrow\)(9x-1,5y)H2O+3NxOy+(5x-2y)Al(NO3)3
xFe+2y/x \(\rightarrow\)xFe+3 + (3x-2y)e .1
N+5 +1e\(\rightarrow\)N+4 ____________ .(3x-2y)
\(\rightarrow\)FexOy+(6x-2y)HNO3\(\rightarrow\)(3x-y)H2O+xFe(NO3)3+(3x-2y)NO2


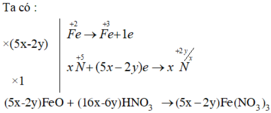
\(\text{(5X-2Y)Fe + (18X-6Y)HNO}_3\rightarrow\text{ }\)(5X-2Y)Fe(NO3)3 + 3NxOy + (9X-3Y)H2O