Bỏ 11,2 gam Fe vào 200 gam dung dịch H2 SO4 19,6%. biết Fe phản ứng hết, C% của dung dịch H2 SO4 sau phản ứng?
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


$a\big)$
$M_A=9,4.2=18,8(g/mol)$
$\to \dfrac{n_{CO_2}}{n_{H_2}}=\dfrac{18,8-2}{44-18,8}=\dfrac{2}{3}$
Mà $n_{CO_2}+n_{H_2}=\dfrac{11,2}{22,4}=0,5(mol)$
\(\begin{array} {l} \to n_{CO_2}=0,2(mol);n_{H_2}=0,3(mol)\\ Fe+2HCl\to FeCl_2+H_2\\ FeCO_3+2HCl\to FeCl_2+CO_2+H_2O\\ \text{Theo PT: }n_{Fe}=n_{H_2}=0,3(mol);n_{FeCO_3}=n_{CO_2}=0,2(mol)\\ \to m=0,3.56+0,2.116=40(g) \end{array}\)
$b\big)$
Đổi $400ml=0,4l$
\(\begin{array} {l} \text{Theo PT: }n_{FeCl_2}=n_{H_2}+n_{CO_2}=0,5(mol)\\ \to C_{M\,FeCl_2}=\dfrac{0,5}{0,4}=1,25M \end{array}\)
$c\big)$
\(\begin{array}{l} m_{dd\,FeCl_2}=\dfrac{400}{1,2}\approx 333,33(g)\\ \to C\%_{FeCl_2}=\dfrac{0,5.127}{333,33}.100\%=19,05\%\end{array}\)

PTHH: \(Fe+H_2SO_4\rightarrow FeSO_4+H_2\uparrow\)
Ta có: \(\left\{{}\begin{matrix}n_{Fe}=\dfrac{33,6}{56}=0,6\left(mol\right)\\n_{H_2SO_4}=\dfrac{784\cdot10\%}{98}=0,8\left(mol\right)\end{matrix}\right.\)
Xét tỉ lệ: \(\dfrac{0,6}{1}< \dfrac{0,8}{1}\) \(\Rightarrow\) H2SO4 còn dư, Fe phản ứng hết
\(\Rightarrow\left\{{}\begin{matrix}n_{FeSO_4}=n_{H_2}=0,6mol\\n_{H_2SO_4\left(dư\right)}=0,2mol\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{FeSO_4}=0,6\cdot152=91,2\left(g\right)\\m_{H_2SO_4\left(dư\right)}=0,2\cdot98=19,6\left(g\right)\\m_{H_2}=0,6\cdot2=1,2\left(g\right)\end{matrix}\right.\)
Mặt khác: \(m_{dd}=m_{Fe}+m_{ddH_2SO_4}-m_{H_2}=816,4\left(g\right)\)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{FeSO_4}=\dfrac{91,2}{816,4}\cdot100\%\approx11,17\%\\C\%_{H_2SO_4\left(dư\right)}=\dfrac{19,6}{816,4}\cdot100\%\approx2,4\%\end{matrix}\right.\)
\(n_{Fe}=\dfrac{33,6}{56}=0,6\left(mol\right)\)
\(n_{H2SO4}=\dfrac{784.10\%}{98}=0,8\left(mol\right)\)
PTHH : \(Fe+H_2SO_4-->FeSO_4+H_2\uparrow\)
Theo pthh : \(n_{H2}=n_{FeSO4}=n_{H2SO4\left(pứ\right)}=n_{Fe}=0,6\left(mol\right)\)
\(\Rightarrow n_{H2SO4\left(dư\right)}=0,8-0,6=0,2\left(mol\right)\)
Áp dụng ĐLBTKL :
mFe + m(dd H2SO4) = m(ddspu) + mH2
=> 33,6 + 784 = m(ddspu) + 0,6.2
=> m(ddspu) = 816,4(g)
\(\Rightarrow\left\{{}\begin{matrix}C\%FeSO_{\text{4}}=\dfrac{0,6.152}{816,4}\cdot100\%\approx11,17\%\\C\%H_2SO_{4\left(dư\right)}=\dfrac{0,2.98}{816,4}\cdot100\%\approx2,4\%\end{matrix}\right.\)

`Fe + H_2 SO_4 -> FeSO_4 + H_2`
`0,25` `0,25` `0,25` `(mol)`
`a)n_[Fe]=[22,4]/56=0,4(mol)`
`n_[H_2 SO_4]=[24,5]/98=0,25(mol)`
Có: `[0,4]/1 > [0,25]/1=>Fe` hết, `H_2 SO_4`
`=>m_[Fe(dư)]=(0,4-0,25).56=8,4(g)`
`b)V_[H_2]=0,25.22,4=5,6(l)`
Ko được ghi `Fe+H_2 SO_4->Fe_2 (SO_4)_3+H_2` vì đây là `H_2 SO_4` loãng

\(n_{Ba}=\dfrac{6,85}{137}=0,05\left(mol\right)\\ m_{H_2SO_4}=500.1,96\%=9,8\left(g\right)\\ PTHH:Ba+H_2SO_4\rightarrow BaSO_4+H_2\uparrow\\ LTL:0,05< 0,1\Rightarrow H_2SO_4.dư\)
\(n_{BaSO_4}=n_{H_2SO_4\left(pư\right)}=n_{Ba}=n_{H_2}=0,05\left(mol\right)\)
\(\Rightarrow n_{H_2SO_4\left(dư\right)}=0,1-0,05=0,05\left(mol\right)\)
\(V_{dd}=\dfrac{500}{1,15}\approx434\left(ml\right)=0,434\left(l\right)\)
\(C_{MBaSO_4}=\dfrac{0,05}{0,434}=0,115M\\ C_{MH_2SO_4\left(dư\right)}=\dfrac{0,05}{0,434}=0,115M\)

a) Số mol của 11,2 gam Fe:
\(n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\)
PTHH:
\(Fe+H_2SO_4\rightarrow FeSO_4+H_2\uparrow\)
1 : 1 : 1 : 1
0,2-> 0,2 : 0,2 : 0,2 (mol)
Khối lượng của 0,2 mol H2:
\(m_{H_2}=n.M=0,2.2=0,4\left(g\right)\)
b) khồi lượng của 0,2 mol H2SO4:
\(m_{H_2SO_4}=n.M=0,2.98=19,6\left(g\right)\)
Nồng độ phần trăm của dd:
\(C\%_{H_2SO_4}=\dfrac{m_{ct}}{m_{dd}}.100\%=\dfrac{19,6}{200}.100\%=9,8\%\)

,nFe2(SO4)3=0.25*1=0.25mol=>nFe3+=0.5 mol
spư thu dc ran z ma ko tan trong H2SO4 loang=>ran Z la Cu,nCu dư=3.28/64=0.05125mol=>Fe tan het
va toan bo Fe(3+) chuyen len het Fe(2+) do Cu dư
dat nFe=x,nCu pư=y=>56x+64y+3.28=17.8 (1)
mat khac theo bt e
Fe=Fe(2+)+2e................Fe(3+)+1e=F...
x-------------> 2x 0.5---->0.5
Cu=Cu(2+)+2e
y------------->2y
vi ne cho=ne nhan=>2x+2y=0.5(2)=>x=0.185,y=0.065
mCu=3.28+0.065*64=7.44g

\(n_{Fe}=\dfrac{16,8}{56}=0,3\left(mol\right)\)
\(Fe+H_2SO_4\rightarrow FeSO_4+H_2\)
\(0,3->0,3-->0,3->0,3\)
\(mH_2SO_4=0,3.98=29,4\left(g\right)\)
\(\Rightarrow C\%_{ddH_2SO_4}=\dfrac{29,4.100}{200}=14,7\%\)
\(V_{FeSO_4}=\dfrac{n}{CM}=\dfrac{0,3}{2}=0,25\left(l\right)\)
\(VH_2=0,3.22,4=6,72\left(l\right)\)
\(n_{Fe}=\dfrac{16,8}{56}=0,3\left(mol\right)\\
pthh:Fe+H_2SO_4\rightarrow FeSO_4+H_2\)
0,3 0,3 0,3 0,3
\(C\%_{H_2SO_4}=\dfrac{0,3.98}{200}.100\%=14,7\%\\
V_{FeSO_4}=\dfrac{0,3}{2}=0,15\left(l\right)\\
V_{H_2}=0,3.22,4=6,72\left(l\right)\)

\(a,n_{Zn}=\dfrac{19,5}{65}=0,3\left(mol\right)\\ m_{H_2SO_4}=200.19,6\%=39,2\left(g\right)\\ \rightarrow n_{H_2SO_4}=\dfrac{39,2}{98}=0,4\left(mol\right)\)
PTHH: \(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\uparrow\)
bđ 0,3 0,4
pư 0,3 0,3
spư 0 0,1 0,3 0,3
\(\rightarrow V_{H_2}=0,3.22,4=6,72\left(l\right)\)
\(b,m_{dd}=19,5+200-0,3.2=218,9\left(g\right)\\ \rightarrow\left\{{}\begin{matrix}C\%_{ZnSO_4}=\dfrac{0,3.161}{218,9}.100\%=22,06\%\\C\%_{H_2SO_4\left(dư\right)}=\dfrac{0,1.98}{218,9}.100\%=4,48\%\end{matrix}\right.\)

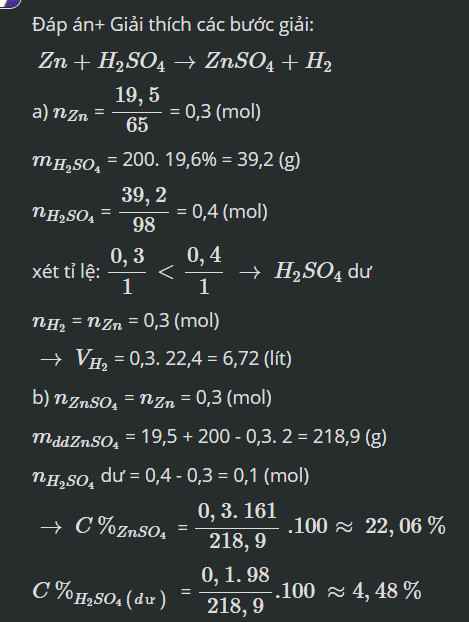
\(Fe+H_2SO_4\rightarrow FeSO_4+H_2\)
\(n_{H_2SO_4\left(bđ\right)}=\dfrac{19,6\%.200}{98}=0,4\left(mol\right)\)
\(n_{Fe}=n_{H_2SO_4\left(pứ\right)}=0,2\left(mol\right)\)
=> \(n_{H_2SO_4\left(dư\right)}=0,4-0,2=0,2\left(mol\right)\)
\(m_{ddsaupu}=11,2+200-0,2.2=210,8\left(g\right)\)
\(C\%_{H_2SO_4\left(dư\right)}=\dfrac{0,2.98}{210,8}.100=9,3\%\)
PTHH: Fe+H2SO4→H2+FeSO4
nFe=\(\dfrac{11,2}{56}\)=0,2(mol)
mH2SO4=\(\dfrac{19,6\%.200}{100\%}\)=39,2(g)
nH2SO4=\(\dfrac{39,2}{98}\)=0,4(mol)
Vì Fe đã pứng hết,theo PTHH ta có:nFe=nH2SO4=0,2(mol)
⇒nH2SO4 dư=0,4-0,2=0,2(mol)
⇒mH2SO4 dư=0,2.98=19,6(g)
m dung dịch sau pứng:200+11.2=211,2(g)
⇒C%H2SO4 sau pứng=\(\dfrac{19,6.100\%}{211,2}\)(xấp xỉ) 9,3%