ai giải giúp mik bài 16 với ạ
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


9 will have to
10 must work
11 will you do - fail
12 will you do
13 were - would buy
14 were - would help
15 had - would travel
16 had - would go
17 would tell - were
18 knew - would speak
19 gets - will go
20 can play - is
21 isn't raining - will have
22 had - would finish
23 wouldn't do - were
24 didn't speak
25 would buy - had
26 weren't - would buy
27 goes
28 will she go
29 would you do - were
30 were - would help
31 would you buy - were
32 would she do - were
33 will they do - become
34 would Ba go - had
35 had been - would have helped
36 had had - would have bought
37 hadn't bought - would have bought
38 would have been - hadn't rained
39 wouldn't have done
40 would have bought
41 would you do
42 would you have done
43 would you have done
44 would you have bought
45 has
46 would have come
III.
1 I will visit you
2 I would travel around the world
3 we will fail the final term test
4 you should take care of her
5 I would run away
6 we will go on a picnic
7 I would have got a good mark
8 he wouldn't be so fat now
9 I would kill it

a: =>x=13/52+8/52=21/52
b: =>x=1/36-27/36=-26/36=-13/18
c: =>x=24/60+15/60-20/60=19/60
d: =>x/15=9/15-10/15=-1/15
=>x=-1

a. Trọng lượng của vật là:
P=10.m= 10.15=150N
Trọng lực có phương thẳng đứng, chiều từ trên xuống dưới.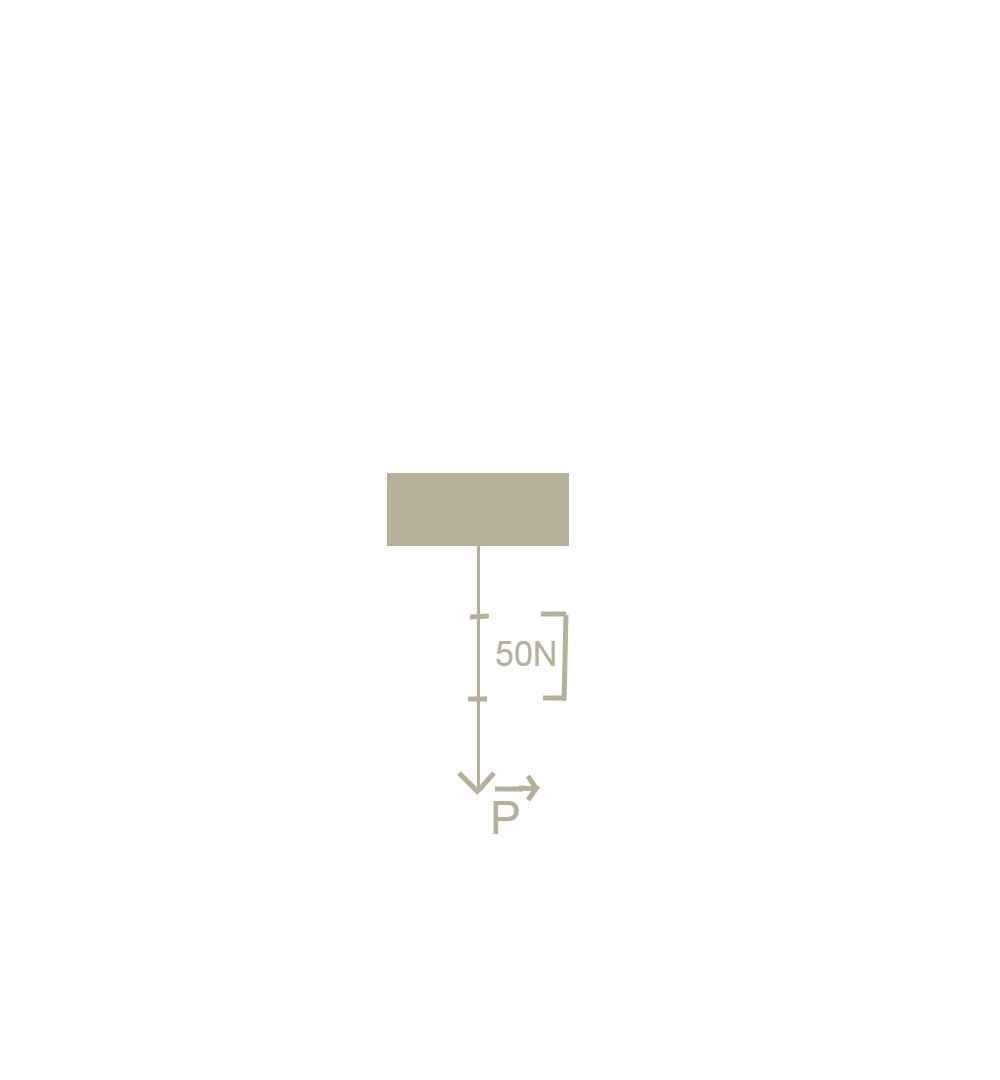 b.
b. 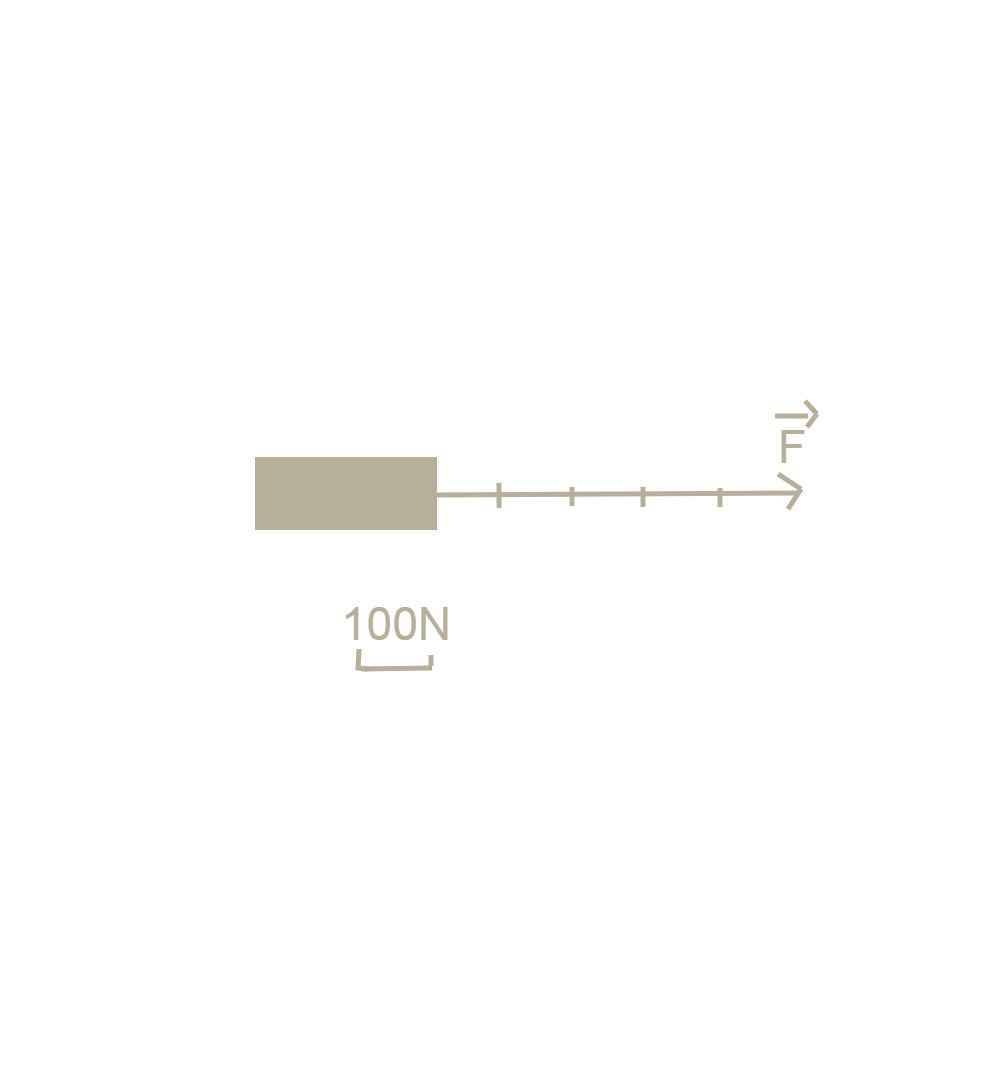 c.Trọng lượng của vật là:
c.Trọng lượng của vật là:
P= 10.m= 10.6=60N
Trọng lượng có phương thẳng, đứng chiều từ trên xuống dưới.
Vì vật đang đứng yên, nên chứng tỏ đã có 2 lực cân bằng tác dụng vào vật. Đó là trọng lực và lực nâng (P = Q)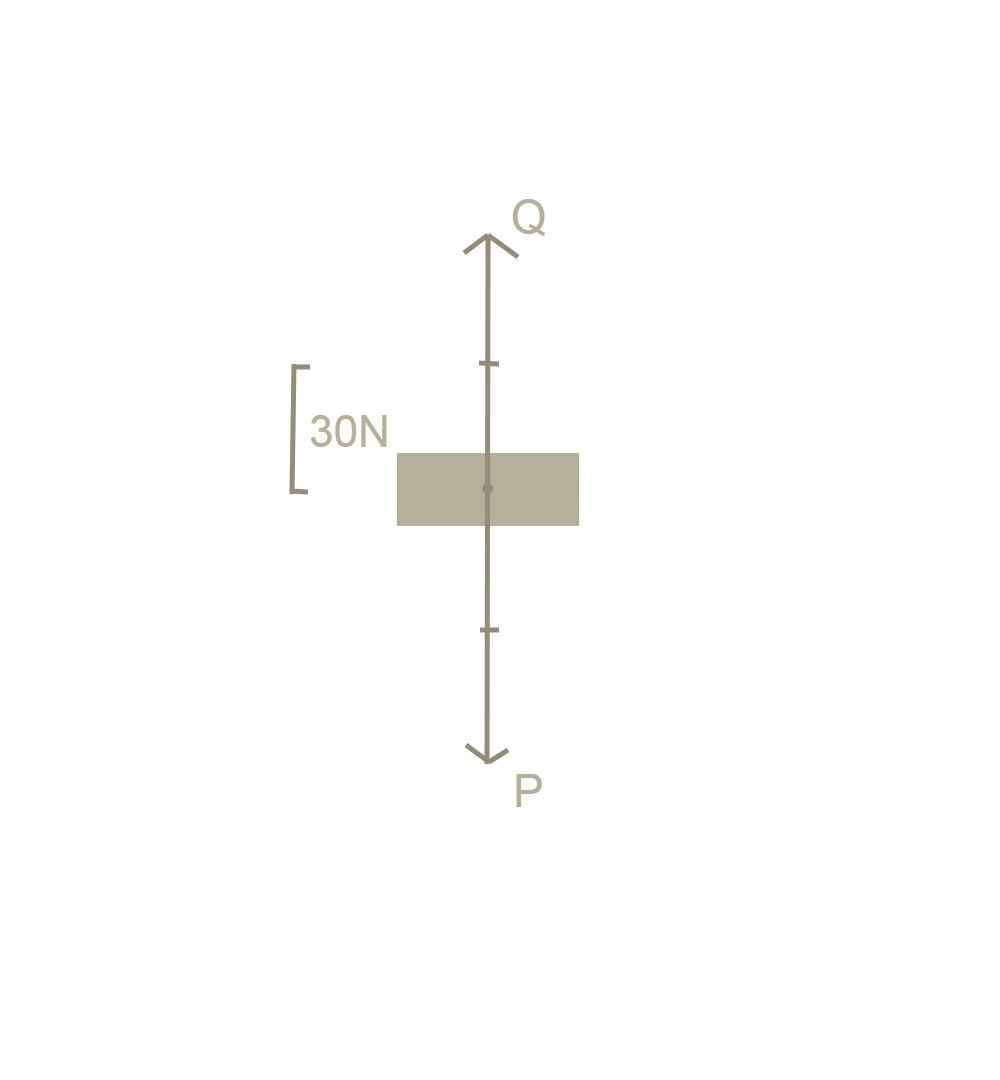

a, ta có A(x)=2x3+7x2+ax+b
=(2x3+2x2+2x)+(5x2+5x+5)+ax-7x+b-5
=2x(x2+x+1)+5(x2+x+1)+(a-7)x+(b-5)
=(x2+x+1)(2x+5)+(a-7)x+(b-5)
ta có: (x2+x+1)(2x+5)⋮B(x)
→để A(x)⋮B(x) thì (a-7)x+(b-5)=0
→\(\left\{{}\begin{matrix}a-7=0\\b-5=0\end{matrix}\right.\) ⇔\(\left\{{}\begin{matrix}a=7\\b=5\end{matrix}\right.\)
vậy ....
mk trình bày hơi tắt xíu
bn cố gắng dịch nhé

Từ giả thiết, suy ra: \(\hat{M}=\dfrac{3}{2}\hat{P}\).
Ta có: \(\hat{D}+\hat{M}+\hat{P}=180^o\) (tổng 3 góc trong một tam giác)
\(\Leftrightarrow55^o+\dfrac{3}{2}\hat{P}+\hat{P}=180^o\Leftrightarrow\hat{P}=50^o\)
\(\Rightarrow\hat{M}=\dfrac{3}{2}\hat{P}=\dfrac{3}{2}\cdot50^o=75^o\)

\(\dfrac{9^{15}.8^{11}}{3^{29}.16^8}=\dfrac{\left(3^2\right)^{15}.\left(2^3\right)^{11}}{3^{29}.\left(2^4\right)^8}=\dfrac{3^{30}.2^{33}}{3^{29}.2^{32}}\)
Ta lấy vễ trên chia vế dưới
\(=3.2=6\)
\(\dfrac{2^{11}.9^3}{3^5.16^2}=\dfrac{2^{11}.\left(3^2\right)^3}{3^5.\left(2^4\right)^2}=\dfrac{2^{11}.3^6}{3^5.2^8}\)
Ta lấy vế trên chia vế dưới
\(=2^3.3=24\)
\(\dfrac{9^{15}.8^{11}}{3^{29}.16^8}=\dfrac{\left(3^2\right)^{15}.\left(2^3\right)^{11}}{3^{29}.\left(2^4\right)^8}=\dfrac{3^{30}.2^{33}}{3^{29}.3^{32}}=3.2=6\)
\(\dfrac{2^{11}.9^3}{3^5.16^2}=\dfrac{2^{11}.\left(3^2\right)^3}{3^5.\left(2^4\right)^2}=\dfrac{2^{11}.3^6}{3^5.2^8}=2^3.3=8.3=24\)

\(\left|2x-3\right|=3-2x\)
\(ĐK:x\le\dfrac{3}{2}\)
\(\Leftrightarrow\left[{}\begin{matrix}2x-3=3-2x\\3-2x=3-2x\end{matrix}\right.\)
\(\Leftrightarrow\left[{}\begin{matrix}x=\dfrac{3}{2}\\0=0\left(đúng\right)\end{matrix}\right.\)
Vậy \(S=\left\{x\in R;x=\dfrac{3}{2}\right\}\)

Câu 3:
a: \(BD=\sqrt{BC^2-DC^2}=4\left(cm\right)\)
b: \(\widehat{A}=180^0-2\cdot70^0=40^0< \widehat{B}\)
nên BC<AC=AB
c: Xét ΔEBC vuông tại E và ΔDCB vuông tại D có
BC chung
\(\widehat{EBC}=\widehat{DCB}\)
Do đó:ΔEBC=ΔDCB
d: Xét ΔOBC có \(\widehat{OBC}=\widehat{OCB}\)
nên ΔOBC cân tại O
Câu 2
a) Thay y = -2 vào biểu thức đã cho ta được:
2.(-2) + 3 = -1
Vậy giá trị của biểu thức đã cho tại y = -2 là -1
b) Thay x = -5 vào biểu thức đã cho ta được:
2.[(-5)² - 5] = 2.(25 - 5) = 2.20 = 40
Vậy giá trị của biểu thức đã cho tại x = -5 là 40

Bài 6:
b: PTHĐGĐ là:
\(x^2+4x-1=x-3\)
\(\Leftrightarrow x^2+3x-4=0\)
\(\Leftrightarrow\left[{}\begin{matrix}x=-4\\x=1\end{matrix}\right.\Leftrightarrow\left[{}\begin{matrix}y=-7\\y=-2\end{matrix}\right.\)

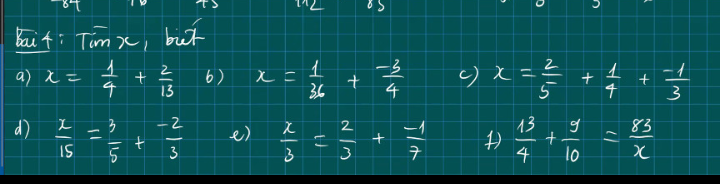

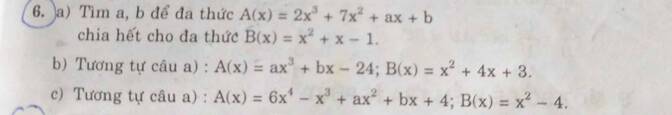


Anh sửa lại chút nha !
\(n_{CO_2}=n_{H_2SO_4}+\dfrac{1}{2}\cdot n_{HCl}=0.4\left(mol\right)\)
\(\Rightarrow n_{HCl}=\left(0.4-0.3\right)\cdot2=0.2\left(mol\right)\)
\(C_{M_{HCl}}=\dfrac{0.2\cdot2}{0.01}=40\left(M\right)\)
\(C_{M_{H_2SO_4}}=\dfrac{0.3\cdot2}{0.01}=60\left(M\right)\)
P1 :
\(BaCl_2+H_2SO_4\rightarrow BaSO_4+2HCl\)
\(n_{BaSO_4}=n_{H_2SO_4}=\dfrac{6.99}{233}=0.3\left(mol\right)\)
P2 :
\(Na_2CO_3+H_2SO_4\rightarrow Na_2SO_4+CO_2+H_2O\)
\(Na_2CO_3+2HCl\rightarrow2NaCl+CO_2+H_2O\)
\(n_{HCl}=0.4-0.3=0.1\left(mol\right)\)
\(C_{M_{HCl}}=\dfrac{0.1\cdot2}{0.01}=20\left(M\right)\)
\(C_{M_{H_2SO_4}}=\dfrac{0.3\cdot2}{0.01}=60\left(M\right)\)