
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(a,V_{H_2\left(25^oC,1bar\right)}=0,1.24,79=2,479\left(l\right)\\ b,V_{CH_4\left(25^oC,1bar\right)}=0,03.24,79=0,7437\left(l\right)\)

\(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\\ a,PTHH:Zn+2HCl\rightarrow ZnCl_2+H_2\\ b,n_{ZnCl_2}=n_{H_2}=n_{Zn}=0,1\left(mol\right)\\ m_{muối}=m_{ZnCl_2}=0,1.136=13,6\left(g\right)\\ V_{khí\left(đktc\right)}=V_{H_2\left(đkc\right)}=0,1.24,79=2,479\left(l\right)\\ c,n_{CuO}=\dfrac{7,6}{80}=0,095\left(mol\right)\\ PTHH:CuO+H_2\rightarrow\left(t^o\right)Cu+H_2O\\ Vì:\dfrac{0,095}{1}< \dfrac{0,1}{1}\Rightarrow H_2dư\\ n_{Cu}=n_{CuO}=0,095\left(mol\right)\\ m_{Cu}=0,095.64=6,08\left(g\right)\)

a, \(2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
b, \(n_{KCl}=\dfrac{0,745}{74,5}=0,01\left(mol\right)\)
Theo PT: \(n_{O_2}=\dfrac{3}{2}n_{KCl}=0,015\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,015.24,79=0,37185\left(l\right)\)
\(m_{O_2}=0,015.32=0,48\left(g\right)\)
c, \(n_{KClO_3\left(pư\right)}=n_{KCl}=0,01\left(mol\right)\)
\(\Rightarrow m_{KClO_3\left(pư\right)}=0,01.122,5=1,225\left(g\right)\)
\(\Rightarrow H=\dfrac{1,225}{2,5}.100\%=49\%\)

\(n_{N_2}=\dfrac{21}{28}=0,75\left(mol\right)\\ \Rightarrow V_{N_2\left(25^oC,1bar\right)}=0,75\cdot24,79=18,5925\left(l\right)\)
Chọn A

a) 4Al + 3O2 --to--> 2Al2O3
b) \(n_{Al}=\dfrac{10,8}{27}=0,4\left(mol\right)\)
PTHH: 4Al + 3O2 --to--> 2Al2O3
0,4-->0,3-------->0,2
VO2(đkc) = 0,3.24,79 = 7,437 (l)
c) mAl2O3 = 0,2.102 = 20,4 (g)

a) $Fe + 2HCl \to FeCl_2 + H_2$
b) Theo PTHH : $n_{FeCl_2} = n_{Fe} = \dfrac{11,2}{56} = 0,2(mol)$
$m_{FeCl_2} = 0,2.127 = 25,4(gam)$
c) $n_{H_2} = n_{Fe} = 0,2(mol)$
$V_{H_2} = 0,2.24,79 = 4,958(lít)$
d) $RO + H_2 \xrightarrow{t^o} R + H_2O$
Theo PTHH : $n_{RO} = n_{H_2} = 0,2(mol)$
$\Rightarrow M_{RO} = R + 16 = \dfrac{16}{0,2} = 80$
$\Rightarrow R = 64(Cu)$
CTHH oxit : $CuO$
$n_{Cu} = n_{H_2} = 0,2(mol) \Rightarrow m_{Cu} = 0,2.64 = 12,8(gam)$

\(n_{CO_2}=\dfrac{48}{44}=1,\left(09\right)\approx1,1\left(mol\right)\)
\(\Rightarrow V_{CO_2\left(dkt\right)}=1,1.24=26,4\left(l\right)\)
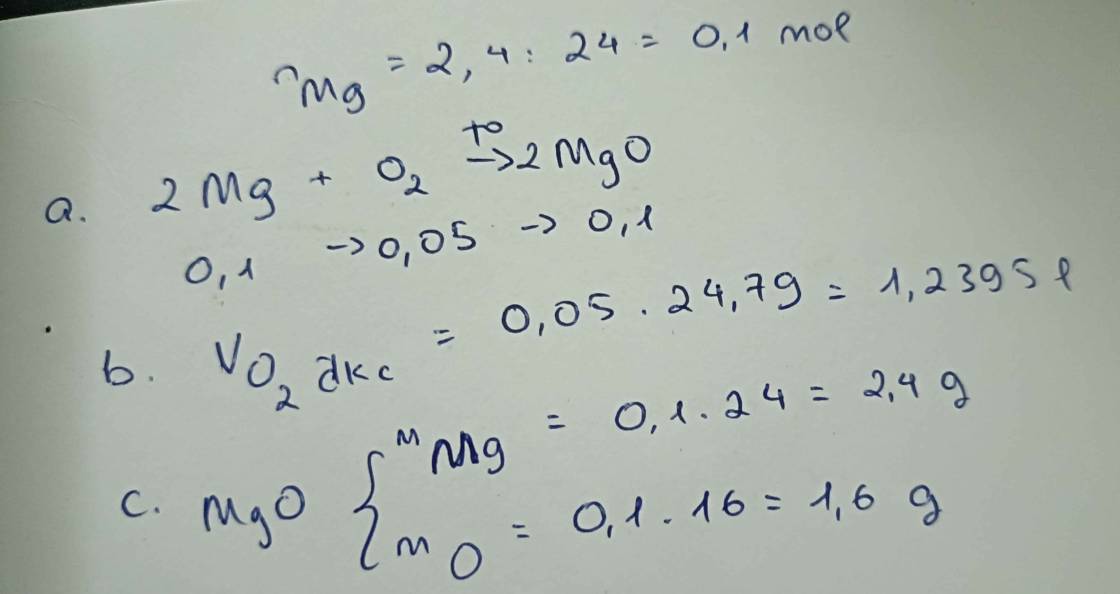
\(n_{H_2S}=\dfrac{17}{34}=0,5\left(mol\right)\\ V_{H_2S\left(25^oC,1bar\right)}=24,79.0,5=12,395\left(l\right)\)