Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a)
nKMnO4 = 47.4/158 = 0.3 (mol)
2KMnO4 -to-> K2MnO4 + MnO2 + O2
0.3_________________________0.15
VO2 = 0.15*22.4 = 3.36 (l)
b)
nKMnO4 = 31.6/158 = 0.2 (mol)
2KMnO4 -to-> K2MnO4 + MnO2 + O2
0.2_________________________0.1
VO2 = 0.1*22.4 = 2.24 (l)
c)
nKMnO4 = 39.5/158 = 0.25 (mol)
2KMnO4 -to-> K2MnO4 + MnO2 + O2
0.25_________________________0.125
VO2 = 0.125*22.4 = 2.8 (l)
2)
a)
nO2 = 3.36/22.4 = 0.15 (mol)
2KMnO4 -to-> K2MnO4 + MnO2 + O2
0.3_________________________0.15
mKMnO4 = 0.3*158 = 47.4(g)
b)
nO2 = 8.96/22.4 = 0.4 (mol)
2KMnO4 -to-> K2MnO4 + MnO2 + O2
0.8_________________________0.4
mKMnO4 = 0.8*158 = 126.4(g)
c)
nO2 = 14.4/32 = 0.45 (mol)
2KMnO4 -to-> K2MnO4 + MnO2 + O2
0.9_________________________0.45
mKMnO4 = 0.9*158 = 142.2(g)

\(n_{Mg}=0,1\left(mol\right)\)
\(2Mg+O_2\underrightarrow{^{to}}2MgO\)
\(\Rightarrow n_{O2}=0,05\left(mol\right);n_{MgO}=0,1\left(mol\right)\)
\(\Rightarrow V_{O2}=0,05.22,4=1,12\left(l\right)\)
\(m_{MgO}=0,1.40=4\left(g\right)\)
\(2KClO_3\underrightarrow{^{to}}2KCl+3O_2\)
\(n_{KClO3}=\frac{0,1}{3}\left(mol\right)\)
\(\Rightarrow_{KClO3}=4,08\left(g\right)\)

Fe2O3 + 3H2 --to--> 2Fe + 3H2O
Fe + 2HCl → FeCl2+ H2
2H2 + O2 --to---> 2H2O
Fe\(_2O_3\)+3CO\(\rightarrow\)2Fe+3CO\(_2\uparrow\)
Fe+H\(_2SO_4\)\(\rightarrow\)\(H_2\uparrow+FeSO_4\)
\(2H_2+O_2\rightarrow2H_2O\) Điều kiện:nhiệt độ
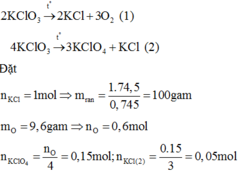
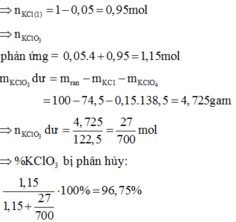
a, Ta có:
\(n_{HgO}=\frac{21,7}{217}=0,1\left(mol\right)\)
\(\Rightarrow n_{O2}=n_{HgO}=0,1\left(mol\right)\)
\(\Rightarrow V_{O2}=0,1.22,4=2,24\left(l\right)\)
b, Ta có:
\(n_{HgO}=0,2\left(mol\right)\Rightarrow n_{Hg}=n_{HgO}=0,2\left(mol\right)\)
\(\Rightarrow m_{Hg}=0,2.201=40,2\left(g\right)\)
c,Ta có:
\(n_{Hg}=0,07\left(mol\right)\Rightarrow n_{HgO}=0,07\left(mol\right)\)
\(\Rightarrow m_{HgO}=0,07.217=15,19\left(g\right)\)
câu c sai