Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


a) Gọi số mol Mg, CuO là a, b (mol)
=> 24a + 80b = 14 (1)
\(n_{HCl}=\dfrac{255,5.10\%}{36,5}=0,7\left(mol\right)\)
PTHH: Mg + 2HCl --> MgCl2 + H2
a--->2a--------->a------>a
CuO + 2HCl --> CuCl2 + H2O
b------>2b----->b
=> 2a + 2b = 0,7 (2)
(1)(2) => a = 0,25 (mol); b = 0,1 (mol)
=> \(\left\{{}\begin{matrix}\%m_{Mg}=\dfrac{0,25.24}{14}.100\%=42,857\%\\\%m_{CuO}=\dfrac{0,1.80}{14}.100\%=57,143\%\end{matrix}\right.\)
b)
mdd sau pư = 14 + 255,5 - 0,25.2 = 269 (g)
\(C\%_{MgCl_2}=\dfrac{0,25.95}{269}.100\%=8,829\%\)
\(C\%_{CuCl_2}=\dfrac{0,1.135}{269}.100\%=5,019\%\)

\(n_{H_2}=\dfrac{6,72}{22,4}=0,3mol\)
\(\left\{{}\begin{matrix}n_{Mg}=x\left(mol\right)\\n_{Zn}=y\left(mol\right)\end{matrix}\right.\Rightarrow24x+65y=11,3\left(1\right)\)
\(Mg+2HCl\rightarrow MgCl_2+H_2\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(\Rightarrow x+y=0,3\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}x=0,2mol\\y=0,1mol\end{matrix}\right.\)
a)\(\%m_{Mg}=\dfrac{0,2\cdot24}{11,3}\cdot100\%=42,48\%\)
\(\%m_{Zn}=100\%-42,48\%=57,52\%\)
b)\(n_{HCl}=2\left(n_{Mg}+n_{Zn}\right)=2\cdot\left(0,2+0,1\right)=0,6mol\)
\(C_{M_{HCl}}=\dfrac{0,6}{0,2}=3M\)

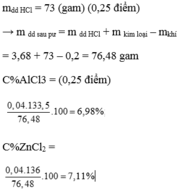
\(\left\{{}\begin{matrix}Fe\\Al\end{matrix}\right.+HCl->\left\{{}\begin{matrix}FeCl2\\AlCl3\end{matrix}\right.+H2\)
Ta có số mol Fe là x , Al là y (mol)
\(\left\{{}\begin{matrix}56x+27y=11\\127x+133,5y=39,4\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=0,1\\y=0,2\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%mFe=\dfrac{0,1.56}{11}=50,9\%\\\%mAl=\dfrac{0,2.27}{11}=49,09\%\end{matrix}\right.\)
Bảo toàn e :
\(2.nH2=2.nFe+3.nAl\Rightarrow nH2=0,4\left(mol\right)\)
\(V=0,4.22,4=8,96\left(l\right)\)
\(nFe=nFeCl2=0,1\left(mol\right)\)
\(nAl=nAlCl3=0,2\left(mol\right)\)
\(\Rightarrow nHCl\left(pứ\right)=2.0,1+3.0,2=0,8\left(mol\right)\)
\(Cm=\dfrac{n}{V}=\dfrac{0,8}{0,2}=4\left(M\right)\)

Đặt :
nFe = x mol
nMgO = y mol
mX = 56x + 40y = 13.6 (g) (1)
Fe + 2HCl => FeCl2 + H2
x____________x
MgO + 2HCl => MgCl2 + H2O
y______________y
mM = mFeCl2 + mMgCl2 = 127x + 95y = 31.7 (2)
(1) , (2) :
x = 0.1
y = 0.2
%Fe = 5.6/13.6 * 100% = 41.17%
%MgO = 58.82%
nKOH = 0.1 * 0.2 = 0.02 (mol)
KOH + HCl => KCl + H2O
0.02____0.02
nHCl (pư) = 2nFe + 2nMgO = 0.1*2 + 0.2*2 = 0.6 (mol)
nHCl = 0.02 + 0.6 = 0.62 (mol)
VddHCl = 0.62/0.5 = 1.24 (M)

\(Đặt:n_{MnO_2}=a\left(mol\right),n_{KMnO_4}=b\left(mol\right)\)
\(m_{hh}=87a+158b=37.96\left(g\right)\left(1\right)\)
\(n_{Cl_2}=\dfrac{10.08}{22.4}=0.45\left(mol\right)\)
\(2KMnO_4+16HCl\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\)
\(MnO_2+4HCl\rightarrow MnCl_2+Cl_2+2H_2O\)
\(n_{Cl_2}=a+2.5b=0.45\left(mol\right)\left(2\right)\)
\(\left(1\right),\left(2\right):a=0.4,b=0.02\)
\(\%MnO_2=\dfrac{0.4\cdot87}{37.96}\cdot100\%=91.68\%\\\%KMnO_4=100-91.68=8.32\% \)
\(m_M=m_{KCl}+m_{MnCl_2}=0.02\cdot74.5+\left(0.4+0.02\right)\cdot126=54.41g\)