Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a,\(n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\)
PTHH: Fe + 2HCl → FeCl2 + H2
Mol: 0,2 0,4 0,2
b, \(V_{H_2}=0,2.22,4=4,48\left(l\right)\)
c, \(m_{HCl}=0,4.36,5=14,6\left(g\right)\)

\(n_{Fe}=\dfrac{11.2}{56}=0.2\left(mol\right)\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(0.2.......0.4....................0.2\)
\(V_{H_2}=0.2\cdot22.4=4.48\left(l\right)\)
\(m_{HCl}=0.4\cdot36.5=14.6\left(g\right)\)
PTHH : \(Fe+2HCl-->FeCl_2+H_2\uparrow\) (1)
\(n_{Fe}=\dfrac{m}{M}=\dfrac{11.2}{56}=0.2\left(mol\right)\)
Từ (1) => \(n_{Fe}=n_{H_2}=0.2\left(mol\right)\)
=> \(V_{H2\left(đktc\right)}=n.22,4=0,2.22,4=4,48\left(l\right)\)
Từ (1) => \(2n_{Fe}=n_{HCl}=0.4\left(mol\right)\)
=> \(m_{HCl}=n.M=0,4.\left(1+35.5\right)=14.6\left(g\right)\)

\(a) Fe + 2HCl \to FeCl_2 + H_2\\ b) n_{FeCl_2} = n_{Fe} =\dfrac{11,2}{56} = 0,2(mol)\\ m_{FeCl_2} = 0,2.127 = 25,4(gam)\\ c) n_{H_2} = n_{Fe} = 0,2(mol)\Rightarrow V_{H_2} = 0,2.22,4 = 4,48(lít)\\ d) n_{HCl} = 2n_{Fe} = 0,4(mol)\\ C\%_{HCl} = \dfrac{0,4.36,5}{300}.100\% = 4,867\%\)

\(n_{Fe}=\dfrac{11,2}{56}=0,2(mol)\\ a,Fe+2HCl\to FeCl_2+H_2\\ b,n_{FeCl_2}=n_{H_2}=n_{Fe}=0,2(mol)\\ \Rightarrow V_{H_2}=0,2.22,4=4,48(l)\\ m_{FeCl_2}=0,2.127=25,4(g)\)

câu 1
\(n_{Fe}=\dfrac{14}{56}=0,25\left(mol\right)\\ pthh:Fe+2HCl\rightarrow FeCl_2+H_2\)
0,25 0,5 0,25 0,25
\(m_{FeCl_2}=0,25.127=31,75g\\
V_{H_2}=0,25.22,4=5,6\\
C_{M\left(HCl\right)}=\dfrac{0,5}{0,2}=2,5M\)
câu 2
1 ) \(m_{\text{dd}}=35+100=135g\\
2,C\%=\dfrac{204}{204+100}.100=60\%\\
=>m\text{dd}=\dfrac{100.204}{60}=340g\)

a) \(Fe+2HCl\rightarrow FeCl_2+H_2\)
b) \(n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\)
Theo PTHH: \(n_{HCl}=2n_{Fe}=0,4\left(mol\right)\)
\(\Rightarrow m_{HCl}=0,4.36,5=14,6\left(g\right)\)
c) Theo PTHH: \(n_{FeCl_2}=n_{H_2}=n_{Fe}=0,2\left(mol\right)\)
\(\Rightarrow m_{FeCl_2}=0,2.127=25,4\left(g\right)\)
d) \(V_{H_2}=0,2.22,4=4,48\left(l\right)\)
e) \(n_{CuO}=\dfrac{24}{80}=0,3\left(mol\right)\)
PTHH: \(CuO+H_2\xrightarrow[]{t^o}Cu+H_2O\)
Xét tỉ lệ: \(\dfrac{0,3}{1}>\dfrac{0,2}{1}\Rightarrow CuO\) dư
Theo PTHH: \(n_{Cu}=n_{H_2}=0,2\left(mol\right)\)
\(\Rightarrow m_{Cu}=0,2.64=12,8\left(g\right)\)

\(nAl=\dfrac{13,5}{27}=0,5\left(mol\right)\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
2 6 2 3 (mol)
0,5 1,5 0,5 0,75 (mol)
a. \(VH_2=0,75.22,4=16,8\left(l\right)\)
b.
\(FeO+H_2\rightarrow Fe+H_2O\)
1 1 1 1 (mol)
0,75 0,75 0,75 0,75
\(m_{Fe}=0,75.42\left(g\right)\)
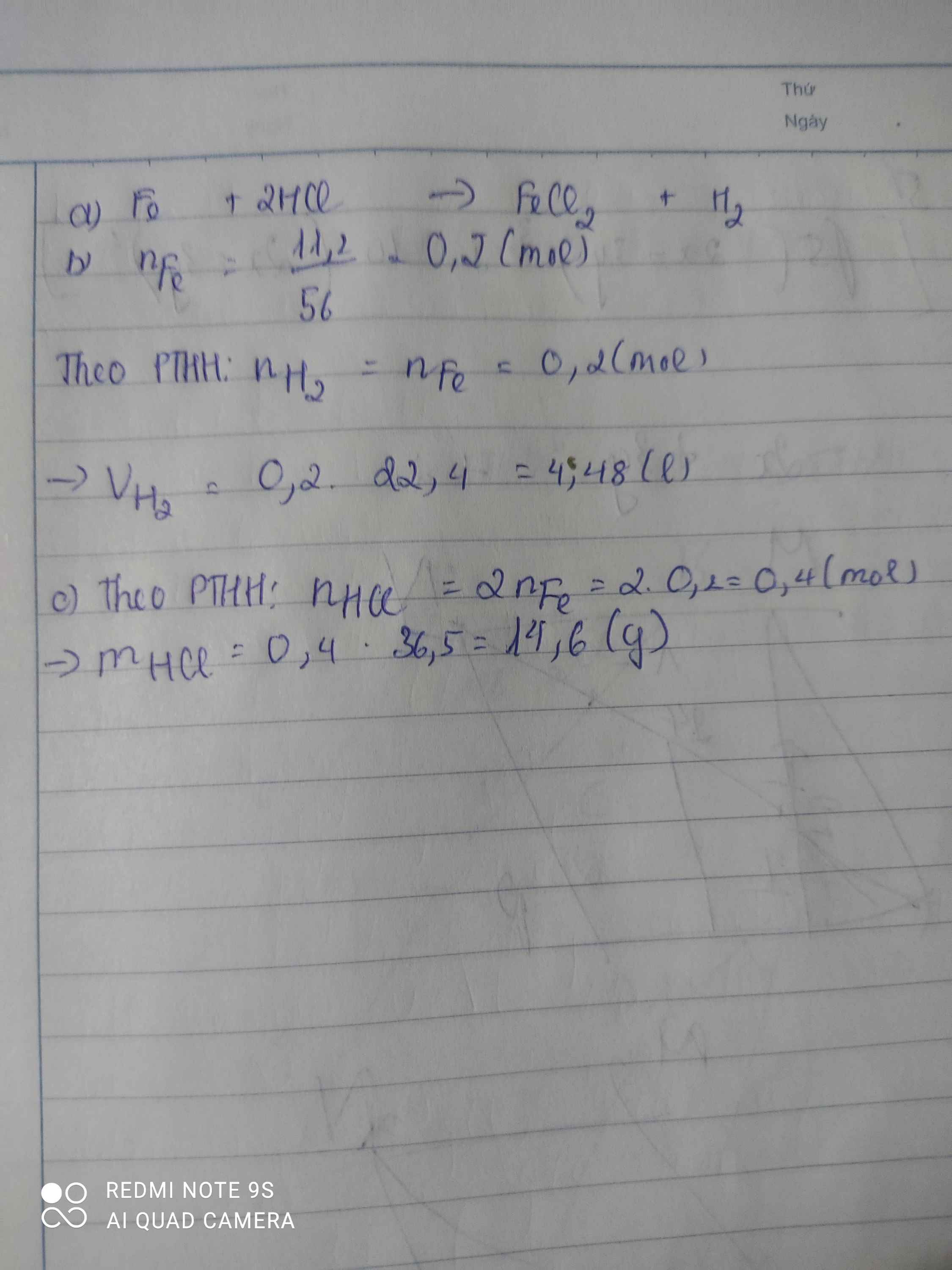

\(a) Fe + 2HCl \to FeCl_2 + H_2\\ n_{FeCl_2} = n_{H_2} = n_{Fe} = \dfrac{5,6}{56} = 0,1(mol)\\ V_{H_2} = 0,1.22,4 = 2,24(lít)\\ b)m_{FeCl_2} = 0,1.127 = 12,7(gam)\\ c) n_{HCl} =2 n_{Fe} = 0,2(mol)\\ C_{M_{HCl}} = \dfrac{0,2}{0,2} = 1M\)
\(n_{Fe}=\dfrac{5.6}{56}=0.1\left(mol\right)\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(0.1......0.2..........0.1..........0.1\)
\(V_{H_2}=0.1\cdot22.4=2.24\left(l\right)\)
\(m_{FeCl_2}=0.1\cdot127=12.7\left(g\right)\)
\(C_{M_{HCl}}=\dfrac{0.2}{0.2}=1\left(M\right)\)