Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{O_2}=\dfrac{V_{O_2}}{24,79}=\dfrac{6,21}{24,79}=0,25mol\)
\(4P+5O_2\rightarrow\left(t^o\right)2P_2O_5\)
0,2 0,25 0,1 ( mol )
\(m_{P_2O_5}=n_{P_2O_5}.M_{P_2O_5}=0,1.142=14,2g\)
\(m_P=n_P.M_P=0,2.31=6,2g\)

\(n_P=\dfrac{12,4}{31}=0,4\left(mol\right)\)
\(n_{O_2}=\dfrac{18}{32}=0,5625\left(mol\right)\)
PTHH :
\(4P+5O_2\rightarrow\left(t^o\right)2P_2O_5\)
0,4 0,5 0,2
\(\dfrac{0,4}{4}< \dfrac{0,5625}{5}\) --> O2 dư sau phản ứng
\(m_{O_2dư}=\left(0,5625-0,5\right).32=2\left(g\right)\)
P2O5 được tạo thành.
\(m_{P_2O_5}=0,2.142=28,4\left(g\right)\)
\(n_P=\dfrac{12,4}{31}=0,4\left(mol\right)\)
\(n_{O_2}=\dfrac{18}{32}=0,5625\left(mol\right)\)
PT: \(4P+5O_2\underrightarrow{t^o}2P_2O_5\)
a, Xét tỉ lệ: \(\dfrac{0,4}{4}< \dfrac{0,5625}{5}\), ta được O2 dư.
Theo PT: \(n_{O_2\left(pư\right)}=\dfrac{5}{4}n_P=0,5\left(mol\right)\Rightarrow n_{O_2\left(dư\right)}=0,0625\left(mol\right)\)
\(\Rightarrow m_{O_2\left(dư\right)}=0,0625.32=2\left(g\right)\)
b, P2O5 được tạo thành.
Theo PT: \(n_{P_2O_5}=\dfrac{1}{2}n_P=0,2\left(mol\right)\)
\(\Rightarrow m_{P_2O_5}=0,2.142=28,4\left(g\right)\)

Ta có: \(n_P=\dfrac{12,4}{31}=0,4\left(mol\right)\)
PT: \(4P+5O_2\underrightarrow{t^o}2P_2O_5\)
_____0,4____0,5_____0,2 (mol)
a, \(m_{P_2O_5}=0,2.142=28,4\left(g\right)\)
b, \(V_{O_2}=0,5.22,4=11,2\left(l\right)\)

PTHH: \(4P+5O_2\underrightarrow{t^o}2P_2O_5\)
a) \(n_P=\dfrac{1,24}{31}=0,04\left(mol\right)\)
Theo PTHH: \(n_{P_2O_5}=\dfrac{0,04\cdot2}{4}=0,02\left(mol\right)\)
\(\Rightarrow m_{P_2O_5}=n_{P_2O_5}\cdot M_{P_2O_5}=0,02\cdot142=2,84\left(g\right)\)
b) Theo PTHH: \(n_{O_2}=\dfrac{0,04\cdot5}{4}=0,05\left(mol\right)\)
\(\Rightarrow V_{O_2\left(dkc\right)}=n_{O_2}\cdot24,79=0,05\cdot24,79=1,2395\left(l\right)\)

\(n_P=\dfrac{12,4}{31}=0,4mol\)
\(n_{O_2}=\dfrac{17}{32}=0,53125mol\)
\(4P+5O_2\rightarrow\left(t^o\right)2P_2O_5\)
\(\dfrac{0,4}{4}\) < \(\dfrac{0,53125}{5}\) ( mol )
0,4 0,5 0,2 ( mol )
\(m_{O_2\left(dư\right)}=\left(0,53125-0,5\right).32=1g\)
\(m_{P_2O_5}=0,2.142=28,4g\)

nP = 24.8 / 31 = 0.8 (mol)
nO2 = 34 / 32 = 1.0625 (mol)
4P + 5O2 -to-> 2P2O5
Bđ: 0.8.....1.0625
Pư: 0.8.........1...............0.4
KT : 0..........0.0625.........0.4
mO2 (dư) = 0.0625 * 32 = 2 (g)
mP2O5 = 0.4 * 142 = 56.8 (g)
PTHH: 4P + 5O2 -\(t^0\) --> 2P2O5
ta có n=m/M
=> nP =0,8 và nO2=2,125
theo pt có
nP/4=0,2 < nO2/5=0,425
=> Oxi dư
theo pt
\(\dfrac{nO2\left(pư\right)}{nP}=\dfrac{5}{4}\Rightarrow nO2\left(pư\right)=\dfrac{5}{4}\cdot0,8=1mol\)
nO2(dư)= 2,125-1=1,125mol
b, chất đc tạo thành là: đi photpho penta oxit
theo pt
\(\dfrac{nP2O5}{nP}=\dfrac{2}{4}\Rightarrow nP2O5=\dfrac{2}{4}\cdot0,8=0,4mol\)
ADCT: m=nM
=> mP2O5=0,4*142=56,8g

a) PTK= 1*2 = 2đvC
b)PTK=1*12+2*16 = 44đvC
c)PTK=1*23+35,5 = 58.5đvC
d)PTK=2*31+5*16 = 142đvC
e)PTK=1*2+4*16 = 66đvC
f)PTK=1*40+1*12+3*16 = 100đvC
a) PTK= 1*2 = 2đvC
b)PTK=1*12+2*16 = 44đvC
c)PTK=1*23+35,5 = 58.5đvC
d)PTK=2*31+5*16 = 142đvC
e)PTK=1*2+4*16 = 66đvC
f)PTK=1*40+1*12+3*16 = 100đvC
g)PTK=2*39+1*32+4*16 = 174đvC
h)PTK=1*27+3*16+1*3 = 78đvC
i)PTK=1*24+2*14+6*16 = 148đvC

\(n_P=\dfrac{6.2}{31}=0.2\left(mol\right)\)
\(n_{O_2}=\dfrac{8.96}{22.4}=0.4\left(mol\right)\)
\(4P+5O_2\underrightarrow{^{^{t^0}}}2P_2O_5\)
\(4........5\)
\(0.2.........0.4\)
Lập tỉ lệ : \(\dfrac{0.2}{4}< \dfrac{0.4}{5}\Rightarrow O_2dư\)
\(n_{P_2O_5}=0.2\cdot\dfrac{2}{4}=0.1\left(mol\right)\)
\(m_{P_2O_5}=0.1\cdot142=14.2\left(g\right)\)
\(m_{P_2O_5\left(tt\right)}=14.2\cdot80\%=11.36\left(g\right)\)
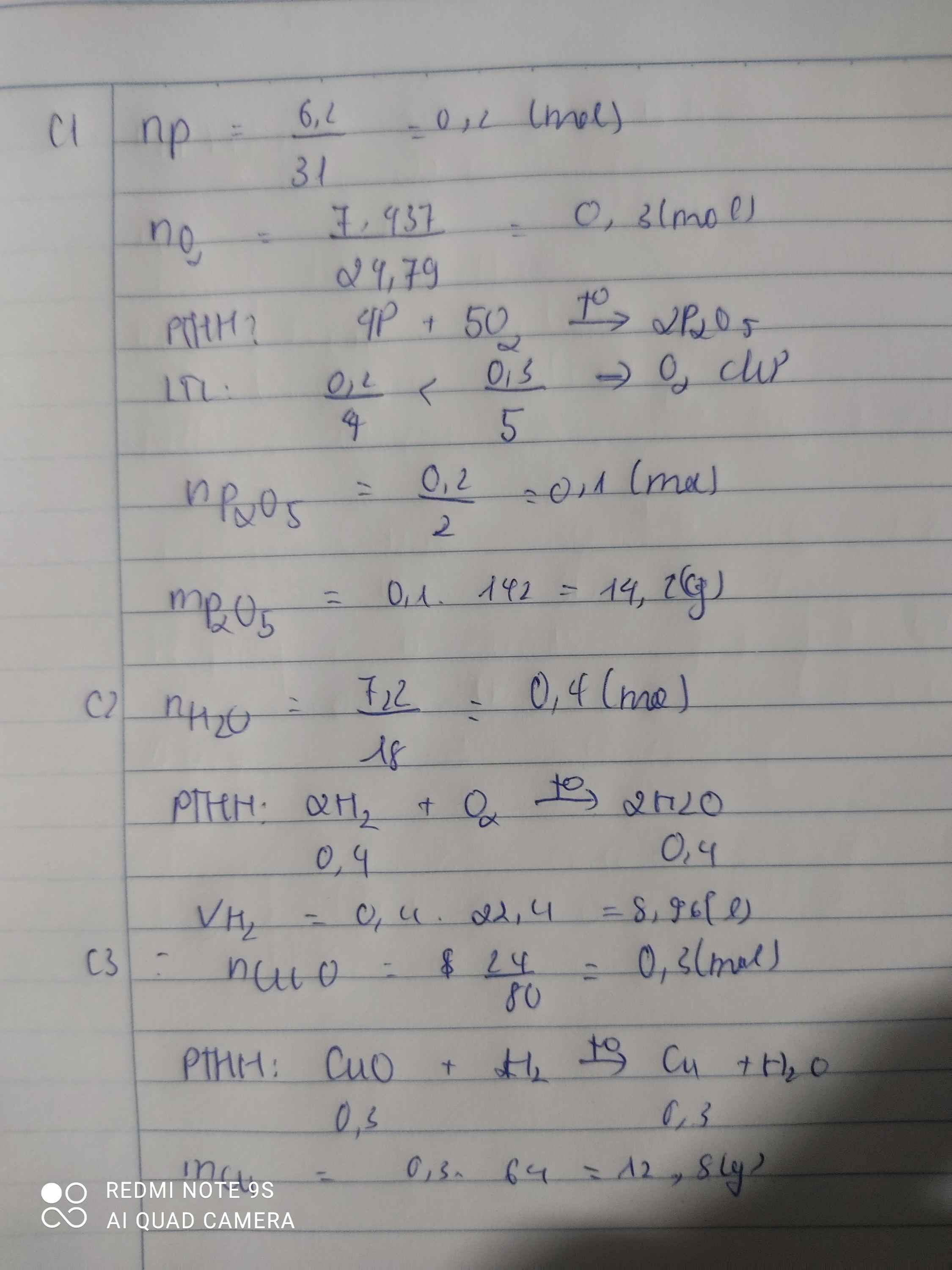
PT: \(4P+5O_2\underrightarrow{t^o}2P_2O_5\)
Ta có: \(n_P=\dfrac{24,8}{31}=0,8\left(mol\right)\)
\(n_{O_2}=\dfrac{40}{32}=1,25\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,8}{4}< \dfrac{1,25}{5}\), ta được O2 dư.
Theo PT: \(n_{P_2O_5}=\dfrac{1}{2}n_P=0,4\left(mol\right)\)
\(\Rightarrow m_{P_2O_5}=0,4.142=56,8\left(g\right)\)