Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a)
$Fe + 2HCl \to FeCl_2 + H_2$
b) Theo PTHH : $n_{Fe} = n_{H_2} = \dfrac{3,36}{22,4} = 0,15(mol)$
$m_{Fe} = 0,15.56 = 8,4(gam)$
c) $n_{HCl} = 2n_{H_2} = 0,3(mol)$
$\Rightarrow C_{M_{HCl}} = \dfrac{0,3}{0,05} = 6M$
d) $2NaOH + H_2SO_4 \to Na_2SO_4 + 2H_2O$
$n_{H_2SO_4} = \dfrac{1}{2}n_{NaOH} = 0,25(mol)$
$m_{dd\ H_2SO_4} = \dfrac{0,25.98}{20\%} = 122,5(gam)$
$V_{dd\ H_2SO_4} = \dfrac{122,5}{1,14} = 107,5(ml)$

a) 2Al + 3H2SO4 --> Al2(SO4)3 + 3H2
b) \(n_{H_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
PTHH: 2Al + 3H2SO4 --> Al2(SO4)3 + 3H2
____\(\dfrac{4}{15}\)<----0,4<--------------------0,4
=> \(m_{Al}=\dfrac{4}{15}.27=7,2\left(g\right)\)
c) \(C_{M\left(H_2SO_4\right)}=\dfrac{0,4}{0,15}=2,667M\)

PTHH: \(Fe+H_2SO_4\rightarrow FeSO_4+H_2\uparrow\)
Ta có: \(n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)=n_{Fe}=n_{H_2SO_4}\)
\(\Rightarrow\left\{{}\begin{matrix}m_{Fe}=0,15\cdot56=8,4\left(g\right)\\C\%_{H_2SO_4}=\dfrac{0,15\cdot98}{150}\cdot100\%=9,8\%\end{matrix}\right.\)

\(Fe+H_2SO_4 \to FeSO_4+H_2\\ n_{H_2}=0,15(mol)\\ a/\\ n_{Fe}=n_{H_2}=0,15(mol)\\ m_{Fe}=0,15.56=8,4(g)\\ b/\\ n_{H_2SO_4}=n_{H_2}=0,15(mol)\\ CM_{H_2SO_4}=\dfrac{0,15}{2}=0,75M c/\\ n_{FeSO_4}=n_{H_2}=0,15(mol)\\ CM_{FeSO_4}=\dfrac{0,15}{0,2}=0,75M\\\)

Tìm số mol Fe tham gia phản ứng :
n Fe = n H = 0,15 mol, suy ra m Fe = 8,4 gam.

\(n_{H2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
a) Pt : \(Fe+2HCl\rightarrow FeCl_2+H_2|\)
1 2 1 1
0,15 0,3 0,15
b) \(n_{Fe}=\dfrac{0,15.1}{1}=0,15\left(mol\right)\)
⇒ \(m_{Fe}=0,15.56=8,4\left(g\right)\)
c) \(n_{HCl}=\dfrac{0,15.2}{1}=0,3\left(mol\right)\)
50ml = 0,05l
\(C_{M_{ddHCl}}=\dfrac{0,3}{0,05}=6\left(M\right)\)
Chúc bạn học tốt

\(Mg+H_2SO_4\rightarrow MgSO_4+H_2\uparrow\\ n_{Mg}=n_{H_2SO_4}=n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\\ a,m_{Mg}=0,15.24=3,6\left(g\right)\\ b,C_{MddH_2SO_4}=\dfrac{0,15}{0,2}=0,75\left(M\right)\)
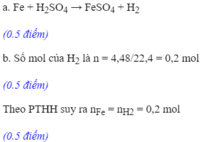

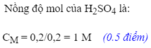
\(n_{H_2}=\dfrac{V_{H_2}}{22,4}=\dfrac{3,36}{22,4}=0,15mol\)
a. PTHH: Fe + H2SO4 \(\rightarrow\) FeSO4 + H2
TL: 1 1 1 1
mol: 0,15 \(\leftarrow\) 0,15 \(\leftarrow\) 0,15 \(\leftarrow\) 0,15
\(b.m_{Fe}=n.M=0,15.56=8,4g\)
Đổi 150ml = 0,15 l
\(c.C_{MddH_2SO_4}=\dfrac{n}{V}=\dfrac{0,15}{0,15}=1M\)