Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, \(Na_2CO_3+2HCl\rightarrow2NaCl+CO_2+H_2O\)
b,\(n_{Na_2CO_3}=\dfrac{21,2}{106}=0,2\left(mol\right)\)
Theo PT: \(n_{HCl}=2n_{Na_2CO_3}=0,4\left(mol\right)\)
\(\Rightarrow m_{HCl}=0,4.36,5=14,6\left(g\right)\)
c, \(n_{CO_2}=n_{Na_2CO_3}=0,2\left(mol\right)\)
\(\Rightarrow V_{CO_2}=0,2.22,4=4,48\left(l\right)\)

Gọi x,y,z lần lượt là số mol của Al,Mg,Zn
PT:
2Al + 6HCl--->2AlCl3 + 3H2
x-------3x----------------------1,5x mol
Mg + 2HCl--->MgCl2 + H2
y-----2y----------------------y mol
Zn + 2HCl--->ZnCl2 + H2
z----2z--------------------z mol
b.
Số mol H2: nH2=16,352/22,4=0,73 mol
1,5x+y+z=0,73
27x = 24y =>x=8y/9
=>7y/3 +z =0,73 (*)
27x + 24y + 65z=19,6
27x = 24y
=> 48y + 65z =19,6 (**)
Từ (*),(**)
=>y=0,27 => mMg =6,48 g
z=0,1=>mZn = 6,5 g
x=0,24=>mAl =6,48g
c.
nHCl =2nH2
=>nHCl =2.0,73=1,46 mol
=>V dd=1,46/2=0,73(l)

PTHH: \(Mg+2HCl\rightarrow MgCl_2+H_2\uparrow\)
a+b+c) Ta có: \(n_{HCl}=\dfrac{18,25}{36,5}=0,5\left(mol\right)\)
\(\Rightarrow n_{H_2}=0,25\left(mol\right)=n_{MgCl_2}\) \(\Rightarrow\left\{{}\begin{matrix}m_{MgCl_2}=0,25\cdot95=23,75\left(g\right)\\V_{H_2}=0,25\cdot22,4=5,6\left(l\right)\end{matrix}\right.\)
d) PTHH: \(CuO+H_2\xrightarrow[]{t^o}Cu+H_2O\)
Theo PTHH: \(n_{Cu}=n_{H_2}=0,25\left(mol\right)\) \(\Rightarrow m_{Cu}=0,25\cdot64=16\left(g\right)\)

a) PTHH: \(Mg+2HCl\rightarrow MgCl_2+H_2\uparrow\)
b) Ta có: \(n_{Mg}=\dfrac{3,6}{24}=0,15\left(mol\right)=n_{MgCl_2}\)
\(\Rightarrow m_{MgCl_2}=0,15\cdot95=14,25\left(g\right)\)
c) Theo PTHH: \(\left\{{}\begin{matrix}n_{HCl\left(p.ứ\right)}=0,3\left(mol\right)\\n_{H_2}=0,15\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{HCl\left(p.ứ\right)}=0,3\cdot36,5=10,95\left(g\right)\\m_{H_2}=0,15\cdot2=0,3\left(g\right)\end{matrix}\right.\)
Mặt khác: \(m_{dd}=m_{Mg}+m_{ddHCl}-m_{H_2}=53,3\left(g\right)\)
\(\Rightarrow m_{ddHCl}=50\left(g\right)\) \(\Rightarrow C\%_{HCl\left(p.ứ\right)}=\dfrac{10,95}{50}\cdot100\%=21,9\%\)

a, Mg + 2HCl \(\rightarrow\) MgCl2 + H2 Cu + 2HCl \(\rightarrow\) CuCl2 + H2
b, \(\left\{{}\begin{matrix}n_{Mg}=x\\n_{Cu}=y\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}24x+64y=16\\x+y=\dfrac{2,24}{22,4}\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=-0,24\\y=0,34\end{matrix}\right.\)
Xem lại đầu bài nha

mHCl= 3,65%.400= 14,6(g) => nHCl=14,6/36,5=0,4(mol)
a) PTHH: Mg +2 HCl -> MgCl2 + H2
0,2__________0,4_____0,2____0,2(mol)
V(H2,đktc)=0,2.22,4=4,48(l)
b)mMg=0,2.24=4,8(g)
c) mMgCl2= 0,2.95=19(g)
mddMgCl2= 400+4,8 - 0,2.2= 404,4(g)
=> C%ddMgCl2= (19/404,4).100=4,698%

a)
\(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
PTHH: Mg + 2HCl --> MgCl2 + H2
_____0,1<---0,2<-------0,1<---0,1
=> mHCl = 0,2.36,5 = 7,3 (g)
=> \(m_{ddHCl}=\dfrac{7,3.100}{7,3}=100\left(g\right)\)
mdd sau pư = 0,1.24 + 100 - 0,1.2 = 102,2 (g)
\(C\%\left(MgCl_2\right)=\dfrac{0,1.95}{102,2}.100\%=9,2955\%\)
b)
CTHH: AaOb
PTHH: \(A_aO_b+2bHCl->aACl_{\dfrac{2b}{a}}+bH_2O\)
____________0,2------->\(\dfrac{0,1a}{b}\)
=> \(\dfrac{0,1a}{b}\left(M_A+35,5.\dfrac{2b}{a}\right)=13,5\)
=> \(M_A=\dfrac{64b}{a}=\dfrac{2b}{a}.32\)
Nếu \(\dfrac{2b}{a}=1\) => MA = 32 (L)
Nếu \(\dfrac{2b}{a}=2\) => MA = 64(Cu)
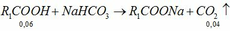
\(n_{HCl}=\dfrac{18.25}{36.5}=0.5\left(mol\right)\)
\(a.Mg+2HCl\rightarrow MgCl_2+H_2\)
\(b.\)
\(n_{Mg}=n_{H_2}=\dfrac{1}{2}\cdot n_{HCl}=\dfrac{1}{2}\cdot0.5=0.25\left(mol\right)\)
\(m_{Mg}=0.25\cdot24=6\left(g\right)\)
\(V_{H_2}=0.25\cdot22.4=5.6\left(l\right)\)
\(c.\)
\(V_{H_2\left(tt\right)}=5.6\cdot90\%=5.04\left(l\right)\)