Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Câu 1:
a, \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
b, \(n_{Zn}=\dfrac{16,25}{65}=0,25\left(mol\right)\)
Theo PT: \(n_{H_2}=n_{Zn}=0,25\left(mol\right)\Rightarrow V_{H_2}=0,25.22,4=5,6\left(l\right)\)
c, \(n_{HCl}=2n_{Zn}=0,5\left(mol\right)\Rightarrow m_{Zn}=0,5.36,5=18,25\left(g\right)\)
Câu 2:
a, \(4P+5O_2\underrightarrow{t^o}2P_2O_5\)
b, \(n_P=\dfrac{1,24}{31}=0,04\left(mol\right)\)
Theo PT: \(n_{O_2}=\dfrac{5}{4}n_P=0,05\left(mol\right)\Rightarrow V_{O_2}=0,05.22,4=1,12\left(l\right)\)
c, \(n_{P_2O_5}=\dfrac{1}{2}n_P=0,02\left(mol\right)\Rightarrow m_{P_2O_5}=0,02.142=2,84\left(g\right)\)

a. \(n_{Zn}=\dfrac{6.5}{65}=0,1\left(mol\right)\)
PTHH : Zn + 2HCl -> ZnCl2 + H2
0,1 0,2 0,1
b. \(V_{H_2}=0,1.22,4=2,24\left(l\right)\)
c. \(m_{HCl}=0,2.36,5=7,3\left(g\right)\)
\(n_{Zn}=\dfrac{6,5}{65}=0,1mol\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
0,1 0,2 0,1
\(V_{H_2}=0,1\cdot22,4=2,24l\)
\(m_{HCl}=0,2\cdot36,5=7,3g\)

\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
a: \(n_{Zn}=\dfrac{52}{65}=0.8\left(mol\right)\)
\(\Leftrightarrow n_{HCl}=1.6\left(mol\right)\)
hay \(n_{H_2}=0.8\left(mol\right)\)
\(V_{H_2}=0.8\cdot22.4=17.92\left(lít\right)\)
b: \(m_{ZnCl_2}=0.8\cdot136=108.8\left(g\right)\)
\(m_{H_2}=0.8\cdot2=1.6\left(g\right)\)
\(n_{Zn}=\dfrac{52}{65}=0,8\left(mol\right)\\ Zn+2HCl\rightarrow ZnCl_2+H_2\\ n_{ZnCl_2}=n_{H_2}=n_{Zn}=0,8\left(mol\right)\\ a,V_{H_2\left(đktc\right)}=0,8.22,4=17,92\left(l\right)\\ b,n_{HCl}=2.0,8=1,6\left(mol\right)\\ C1:m_{ZnCl_2}=0,8.136=108,8\left(g\right);m_{H_2}=0,8.2=1,6\left(g\right)\\ \Rightarrow m_{thu.được}=m_{ZnCl_2}+m_{H_2}=108,8+1,6=110,4\left(g\right)\\ C2:m_{HCl}=1,6.36,5=58,4\left(g\right)\\ \Rightarrow m_{thu.được}=m_{tham.gia}=m_{Zn}+m_{HCl}=52+58,4=110,4\left(g\right)\)

a) Số mol kẽm tham gia phản ứng : \(n_{Zn}=\dfrac{m_{Zn}}{M_{Zn}}=\dfrac{16,25}{65}=0,25\left(mol\right)\).
PTHH : \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
Mol : 1 : 2 : 1 : 1
Mol : 0,25 → 0,5 → 0,25 → 0,5
Suy ra, số mol dung dịch Axit Clohidric \(HCl\) tham gia phản ứng là \(n_{HCl}=0,5\left(mol\right)\).
Khối lượng dung dịch đã dùng : \(m_{HCl}=n_{HCl}.M_{HCl}=\left(0,5\right).\left(36,5\right)=18,25\left(g\right)\).
b) Từ câu a, suy ra số mol khí Hidro sinh ra là \(n_{H_2}=0,25\left(mol\right)\).
Thể tích khí Hydro sinh ra là : \(V_{H_2}=n_{H_2}.22,4=\left(0,25\right).\left(22,4\right)=5,6\left(l\right)\)

\(n_{HCl}=0,25.2=0,5\left(mol\right)\\ a,PTHH:Zn+2HCl\rightarrow ZnCl_2+H_2\\ b,n_{Zn}=n_{H_2}=n_{ZnCl_2}=\dfrac{0,5}{2}=0,25\left(mol\right)\\ m_{Zn}=0,25.65=16,25\left(g\right)\\ c,V_{H_2\left(đktc\right)}=0,25.22,4=5,6\left(l\right)\)
Không biết đúng không nữa;-;;;
a) PTHH: Zn + 2HCl -> ZnCl2 + H2
b) HCl=250ml=0,25l
n2HCl= V/22,4= 0,5/22,4= 0,02(mol)
Zn + 2HCl -> ZnCl2 + H2
1 2 1 1
0,01 <-0,5--------------> 0,01
mZn= n.M= 0,01.65= 0,65(gam)
c) VH2=n . 22,4= 0,01 . 22,4= 0,224(l)

\(n_{Zn}=\dfrac{19,5}{65}=0,3\left(mol\right)\)
PTHH :
\(Zn+2HCl\rightarrow ZnCl_2+H_2\uparrow\)
0,3 0,6 0,3 0,3
\(a,V_{H_2}=n.22,4=0,3.22,4=6,72\left(l\right)\)
\(m_{ZnCl_2}=0,3.136=40,8\left(g\right)\)
\(b,V_{ddHCl}=\dfrac{n}{C_M}=\dfrac{0,6}{2}=0,3\left(l\right)\)
\(c,Fe_2O_3+3H_2\rightarrow2Fe+3H_2O\)
0,1 0,3
\(n_{Fe_2O_3}=\dfrac{16}{160}=0,1\left(mol\right)\)
Ta có :
\(\dfrac{0,1}{1}=\dfrac{0,3}{3}\)
nên không chất nào dư

\(n_{Zn}=\dfrac{26}{65}=0,4\left(mol\right)\\ pthh:Zn+2HCl\rightarrow ZnCl_2+H_2\uparrow\)
0,4 0,8 0,4 0,4
\(a,V_{H_2}=0,4.22,4=8,96\left(l\right)\\ b,C\%_{HCl}=\dfrac{0,8.36,5}{150}.100\%=19,5\%\\ c,m_{\text{dd}}=26+150-\left(0,4.2\right)=175,2\left(g\right)\\ C\%_{ZnCl_2}=\dfrac{0,4.136}{175,2}.100\%=31\%\)

2Al+6HCl--->2AlCl3+3H2
x------3x
Zn+2HCl--->ZnCl2+H2
y-------2y
Ta có
n HCl=36,5/36,5=1(mol)
Theo bài ta có hệ pt
\(\left\{{}\begin{matrix}27x+65y=18,4\\3x+2y=1\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=0,2\\y=0,2\end{matrix}\right.\)
mAl=0,2.27=5,4(g)
mZn=0,2.65=13(g)
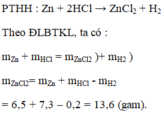
\(PTHH:Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
Theo ĐLBTKL:
mZn + mHCl = mH2 + mZnCl2
=> mHCl = 40,8 + 0,3.2 - 19,5 = 21,9(g)