Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Bài này mình đã làm rồi và được thầy kiểm tra đúng nhé : https://hoc24.vn/cau-hoi/cho-1505-g-hon-hop-a-gom-natri-oxit-va-natri-tac-dung-voi-nuoc-thu-duoc-250-ml-dungdich-natrihidroxit-va-28-lit-khi-dktca-viet-phuong-trinh-hoa-hoc-cho-phan-ung-xay-rab-tinh-nong-do-mol-cua-du.850364428404
Bạn chú ý để tránh đăng câu hỏi lặp nhé
a)
2Na+2H2O→2NaOH+H22Na+2H2O→2NaOH+H2
Na2O+H2O→2NaOHNa2O+H2O→2NaOH
b)
n H2 = 2,8.22,4 = 0,125(mol)
=> n Na = 2n H2 = 0,125.2 =0,25(mol)
=> n Na2O = (15,05 - 0,25.23)/62 = 0,15(mol)
=> n NaOH = n Na + 2n Na2O = 0,55(mol)
=> CM NaOH = 0,55/0,25 = 2,2M
c)
%m Na = 0,25.23/15,05 .100% = 38,21%
%m Na2O = 100% -38,21% = 61,79%
d)
CO2+NaOH→NaHCO3CO2+NaOH→NaHCO3
n CO2 = n NaOH = 0,55(mol)
=> V CO2 = 0,55.22,4 = 12,32(lít)

a,\(n_{Na}=\dfrac{4,6}{23}=0,2\left(mol\right);n_K=\dfrac{3,9}{39}=0,1\left(mol\right)\)
PTHH: 2Na + 2H2O → 2NaOH + H2
Mol: 0,2 0,1
PTHH: 2K + 2H2O → 2KOH + H2
Mol: 0,1 0,05
b, \(n_{H_2}=0,1+0,05=0,15\left(mol\right)\)
\(V_{H_2}=0,15.22,4=3,36\left(l\right)\)
c,mdd sau pứ=4,6+3,9+91,5-0,15.2=99,7 (g)
\(\%m_{NaOH}=\dfrac{0,2.40.100\%}{99,7}=8,02\%\)
\(\%m_{KOH}=\dfrac{0,1.56.100\%}{99,7}=5,62\%\)
Bài 3 :
\(n_{Na}=\dfrac{4,6}{23}=0,2\left(mol\right)\)
\(n_K=\dfrac{3,9}{39}=0,1\left(mol\right)\)
a) Pt : \(2Na+2H_2O\rightarrow2NaOH+H_2|\)
2 2 2 1
0,2 0,2 0,1
\(2K+2H_2O\rightarrow2KOH+H_2|\)
2 2 2 1
0,1 0,1 0,05
b) \(n_{H2\left(tổng\right)}=0,1+0,05=0,15\left(mol\right)\)
\(V_{H2\left(dktc\right)}=0,15.22,4=3,36\left(l\right)\)
c) \(n_{NaOH}=\dfrac{0,1.2}{1}=0,2\left(mol\right)\)
⇒ \(m_{NaOH}=0,2.40=8\left(g\right)\)
\(n_{KOH}=\dfrac{0,05.2}{1}=0,1\left(mol\right)\)
⇒ \(m_{KOH}=0,1.56=5,6\left(g\right)\)
\(m_{ddspu}=8,5+91,5-\left(0,15.2\right)=99,7\left(g\right)\)
\(C_{NaOH}=\dfrac{8.100}{99,7}=8,02\)0/0
\(C_{KOH}=\dfrac{5,6.100}{99,7}=5,62\)0/0
Chúc bạn học tốt

\(n_{Na_2O}=\dfrac{1.86}{62}=0.03\left(mol\right)\)
\(Na_2O+H_2O\rightarrow2NaOH\)
\(0.03........................0.06\)
\(C_{M_{NaOH}}=\dfrac{0.06}{0.25}=0.24\left(M\right)\)
\(2NaOH+CO_2\rightarrow Na_2CO_3+H_2O\)
\(0.06...........0.03\)
\(V_{CO_2}=0.03\cdot22.4=0.672\left(l\right)\)

\(a) Fe + 2HCl \to FeCl_2\\ b) n_{HCl} = \dfrac{182,5.5\%}{36,5} = 0,25(mol)\\ n_{FeCl_2} = n_{H_2} = n_{Fe} = \dfrac{1}{2}n_{HCl} = 0,125(mol)\\ \Rightarrow m_{Fe} = 0,125.56 = 7(gam) ; V = 0,125.22,4 = 2,8(lít)\\ c) m_{dd\ sau\ phản\ ứng} = m_{Fe} + m_{dd\ HCl} - m_{H_2} = 7 + 182,5 - 0,125.2 = 189,25(gam)\\ C\%_{FeCl_2} = \dfrac{0,125.127}{189,25}.100\% = 8,39\%\)

Fe+2HCl->FeCl2+H2
0,125--0,25---0,125-0,125
m HCl=9,125 g=>n HCl=\(\dfrac{9,125}{26,5}\)=0,25 mol
=>m Fe=0,125.56=7g
=>VH2=0,125.22,4=2,8l
=>C%FeCl2=\(\dfrac{0,125.127}{7+182,5-0,25}\).100=8,388%

\(n_{H_2}=\dfrac{5,6}{22,4}=0,25(mol)\\ a,PTHH:Fe+2HCl\to FeCl_2+H_2\\ 2Al+6HCl\to 2AlCl_3+3H_2\)
\(b,\) Đặt \(n_{Fe}=x(mol);n_{Al}=y(mol)\)
\(\Rightarrow 56x+27y=8,3(1)\)
Theo PTHH: \(x+1,5y=0,25(2)\)
\((1)(2)\Rightarrow x=y=0,1(mol)\\ \Rightarrow \%_{Fe}=\dfrac{0,1.56}{8,3}.100\%=67,47\%\\ \%_{Al}=100\%-67,47\%=32,53\%\)

`a)PTHH:`
`2Al + 6HCl -> 2AlCl_3 + 3H_2`
`0,2` `0,6` `0,3` `(mol)`
`n_[Al]=[5,4]/27=0,2(mol)`
`b)V_[H_2]=0,3.22,4=6,72(l)`
`c)m_[dd HCl]=[0,6.36,5]/10 . 100 =219(g)`

a) PTHH: Na2O + H2O \(\rightarrow\) 2NaOH
b) n\(Na_2O\) = \(\frac{12,4}{62}=0,2\left(mol\right)\)
Theo PT: nNaOH =2n\(Na_2O\) =2.0,2=0,4 (mol)
=> CM NaOH = \(\frac{0,4}{0,25}=1,6\left(M\right)\)
c) PTHH: CO2 + 2NaOH \(\rightarrow\) Na2CO3 + H2O
Theo PT: n\(CO_2\) = \(\frac{1}{2}n_{NaOH}=\frac{1}{2}.0,4=0,2\left(mol\right)\)
=> V\(CO_2\) = 0,2.22,4 = 4,48 (l)
nNa2O= 12,4/62= 0,2mol
Na2O +H20----> 2NaOH
pt: 1mol 2mol
đb: 0,2mol ?
theo pt: nNa2O= 1/2nNaOH
=) nNaOH= 0.2.2= 0,4mol
250ml= 0,25l
CMNaOH= 0,4/0,25=
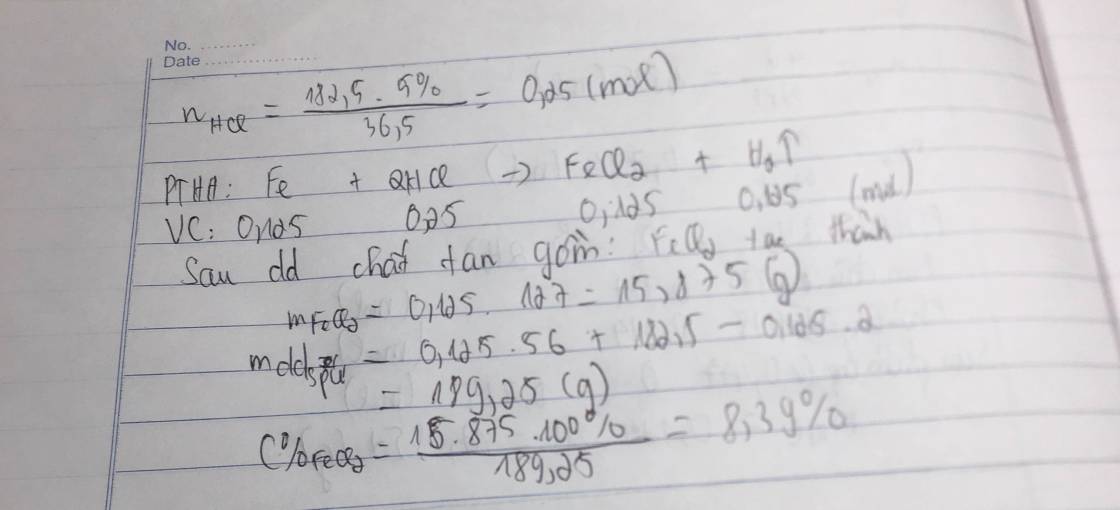
a)
$2Na + 2H_2O \to 2NaOH + H_2$
$Na_2O + H_2O \to 2NaOH$
b)
n H2 = 2,8.22,4 = 0,125(mol)
=> n Na = 2n H2 = 0,125.2 =0,25(mol)
=> n Na2O = (15,05 - 0,25.23)/62 = 0,15(mol)
=> n NaOH = n Na + 2n Na2O = 0,55(mol)
=> CM NaOH = 0,55/0,25 = 2,2M
c)
%m Na = 0,25.23/15,05 .100% = 38,21%
%m Na2O = 100% -38,21% = 61,79%
d)
$CO_2 + NaOH \to NaHCO_3$
n CO2 = n NaOH = 0,55(mol)
=> V CO2 = 0,55.22,4 = 12,32(lít)