 giúp em với ạ em đang cần gấp ạ. Bài nào làm đc trc thì làm trc giúp em với ạ
giúp em với ạ em đang cần gấp ạ. Bài nào làm đc trc thì làm trc giúp em với ạ
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


Câu 2.
\(C=\dfrac{N}{20}=\dfrac{4080}{20}=204\)(chu kì)
\(L=\dfrac{N}{2}\cdot3,4=\dfrac{4080}{2}\cdot3,4=6936A^o\)
\(G=X=30\%\cdot4080=1224nu\)
\(A=T=\dfrac{4080-2\cdot1224}{2}=816nu\)

7. How often does he go to the library?
8. ....is the shortest student in his class.
9......is her address?
10... is shorter than Ba.

Bài 6:
\(\Leftrightarrow6n+4⋮2n-1\)
\(\Leftrightarrow2n-1\in\left\{1;-1;7;-7\right\}\)
hay \(n\in\left\{1;0;4;-3\right\}\)

\(a,x+\dfrac{1}{2}=\dfrac{3}{4}\\ x=\dfrac{3}{4}-\dfrac{1}{2}\\ x=\dfrac{1}{2}\\ b,-\dfrac{2}{3}-x=1\\x=-\dfrac{2}{3}-1\\ x=-\dfrac{5}{3}\\ d,\dfrac{1}{4}+\dfrac{3}{4}:x=\dfrac{5}{2}\\ \dfrac{3}{4}:x=\dfrac{5}{2}-\dfrac{1}{4}\\ \dfrac{3}{4}:x=\dfrac{9}{4}\\ x=\dfrac{3}{4}:\dfrac{9}{4}\\ x=\dfrac{1}{3}\\ e,\left(x+\dfrac{1}{4}\right)\cdot\dfrac{3}{4}=-\dfrac{5}{8}\\ x+\dfrac{1}{4}=-\dfrac{5}{8}:\dfrac{3}{4}\\ x+\dfrac{1}{4}=\dfrac{5}{6}\\ x=\dfrac{5}{6}-\dfrac{1}{4}\\ x=\dfrac{7}{12}\)
\(g,\dfrac{x-3}{15}=\dfrac{-2}{5}\\ 5\left(x-3\right)=-30\\ x-3=-6\\ x=-6+3\\ x=-3\\ h,\dfrac{x}{-2}=\dfrac{-8}{x}\\ x^2=16\\ x=\pm\sqrt{16}\\ x=\pm4\\ k,\dfrac{x+2}{3}=\dfrac{x-4}{5}\\ 5\left(x+2\right)=3\left(x-4\right)\\ 5x+10=3x-12\\ 5x-3x=-12-10\\ 2x=-22\\ x=-11\)
\(m,\left(2x-1\right)^2=4\\ \Rightarrow\left[{}\begin{matrix}2x-1=2\\2x-1=-2\end{matrix}\right.\Rightarrow\left[{}\begin{matrix}2x=3\\2x=-1\end{matrix}\right.\\ \Rightarrow\left[{}\begin{matrix}x=\dfrac{3}{2}\\x=-\dfrac{1}{2}\end{matrix}\right.\)

Câu 7:
a, \(Fe+H_2SO_4\rightarrow FeSO_4+H_2\)
\(CuO+H_2SO_4\rightarrow CuSO_4+H_2O\)
b, \(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
Theo PT: \(n_{Fe}=n_{H_2}=0,1\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Fe}=\dfrac{0,1.56}{10}.100\%=56\%\\\%m_{CuO}=44\%\end{matrix}\right.\)
c, \(n_{CuO}=\dfrac{10-0,1.56}{80}=0,055\left(mol\right)\)
Theo PT: \(n_{H_2SO_4}=n_{Fe}+n_{CuO}=0,155\left(mol\right)\)
\(\Rightarrow C\%_{H_2SO_4}=\dfrac{0,155.98}{100}.100\%=15,19\%\)
d, Theo PT: \(\left\{{}\begin{matrix}n_{FeSO_4}=n_{Fe}=0,1\left(mol\right)\\n_{CuSO_4}=n_{CuO}=0,055\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}m_{FeSO_4}=0,1.152=15,2\left(g\right)\\m_{CuSO_4}=0,055.160=8,8\left(g\right)\end{matrix}\right.\)
Câu 8:
a, \(CuCO_3+2HCl\rightarrow CuCl_2+CO_2+H_2O\)
b, \(n_{CO_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
Theo PT: \(n_{CuCO_3}=n_{CO_2}=0,15\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{CuCO_3}=\dfrac{0,15.124}{20}.100\%=93\%\\\%m_{CuCl_2}=7\%\end{matrix}\right.\)
c, \(n_{HCl}=2n_{CO_2}=0,3\left(mol\right)\)
\(\Rightarrow C_{M_{HCl}}=\dfrac{0,3}{0,2}=1,5\left(M\right)\)


 giúp em 3 bài này với ạ em đang cần gấp chiều em hc ròi ạ ai làm đc bài nào thì gửi luôn giúp em ạ
giúp em 3 bài này với ạ em đang cần gấp chiều em hc ròi ạ ai làm đc bài nào thì gửi luôn giúp em ạ

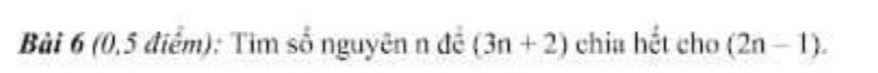 mn giúp em với ạ, em đang cần gấp lắm ạ
mn giúp em với ạ, em đang cần gấp lắm ạ giúp em với ạ ai làm đc thì em xin cảm ơn ạ em đang cần gấp được ko ạ
giúp em với ạ ai làm đc thì em xin cảm ơn ạ em đang cần gấp được ko ạ





6:
\(2^{225}=\left(2^3\right)^{75}=8^{75}\)
\(3^{150}=\left(3^2\right)^{75}=9^{75}\)
mà 8<9
nên \(2^{225}< 3^{150}\)
4: \(\left|5x+3\right|>=0\forall x\)
=>\(-\left|5x+3\right|< =0\forall x\)
=>\(-\left|5x+3\right|+5< =5\forall x\)
Dấu = xảy ra khi 5x+3=0
=>x=-3/5
1:
\(\left(2x+1\right)^4>=0\)
=>\(\left(2x+1\right)^4+2>=2\)
=>\(M=\dfrac{3}{\left(2x+1\right)^4+2}< =\dfrac{3}{2}\)
Dấu = xảy ra khi 2x+1=0
=>x=-1/2